Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the following pairs of species is not a conjugate acid-base pair?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
B
Which of the following substances is never a Brønsted-Lowry acid in an aqueous solution?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
Calculate the pH of a 0.09 M solution of ascorbic acid (Ka1 = 7.9 × 10-5; Ka2 is 1.6 × 10-12).
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
B
Which is the stronger Brønsted-Lowry acid,Fe(H2O)62+ or Fe(H2O)63+? Explain.
(Essay)
4.8/5  (30)
(30)
Which of the following species is amphiprotic in aqueous solution?
(Multiple Choice)
4.8/5  (26)
(26)
Which equation depicts the hydrogen phosphate ion behaving as a Brønsted-Lowry base in water?
(Multiple Choice)
4.9/5  (34)
(34)
What is the pOH of 0.074 M HI(aq)at 25 °C? (Kw = 1.01 × 10-14)?
(Multiple Choice)
4.9/5  (37)
(37)
What is the pH of a solution prepared by dissolving 0.459 L of HCl(g),measured at STP,in enough water such that the total volume of the solution is 5.50 L? (R = 0.0821 L · atm/K·mol)
(Multiple Choice)
4.8/5  (38)
(38)
What is the equilibrium constant for the following reaction, HCO2H(aq)+ CN-(aq) 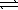 HCO2-(aq)+ HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 × 10-4 and the acid dissociation constant for HCN is 4.0 × 10-10.
HCO2-(aq)+ HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 × 10-4 and the acid dissociation constant for HCN is 4.0 × 10-10.
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following ionic compounds forms a neutral aqueous solution at 25 °C?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is the strongest acid in aqueous solution?
(Multiple Choice)
4.8/5  (26)
(26)
What is the equilibrium pH of a 0.700 M solution of H3PO4(aq)? (Ka1 = 7.5 × 10-3,Ka2 = 6.2 × 10-8,Ka3 = 4.8 × 10-13)
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the pH of the following aqueous solution: 0.17 M H2S (pKa1 = 7.00; pKa2 = 12.89)
(Multiple Choice)
4.8/5  (33)
(33)
The H3O+ concentration of a solution is 5.9 × 10-6 M.What is the pH of the solution?
(Multiple Choice)
5.0/5  (48)
(48)
Which equation depicts aqueous hydrogen sulfide behaving as a Brønsted-Lowry base in water?
(Multiple Choice)
4.8/5  (43)
(43)
What volume of water must be added to 14.4 mL of a pH 2.0 solution of HNO3 in order to change the pH to 4.0?
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following equations shows that isoquinoline,C9H7N,behaves as a Brønsted-Lowry base in water?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 1 - 20 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)