Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
The standard free energy of formation of AgI(s)is -66.2 kJ/mol.ΔrG° for the reaction 2AgI(s)→ 2Ag(s)+ I2(s)is:
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
Which of the following represents the change in entropy for a system going from 142 possible microstates to 830 possible microstates? (k = 1.381 × 10-23 J/K)
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
D
At what temperatures will a reaction be spontaneous if ΔrH° = +117 kJ and ΔrS° = -35 J/K?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
E
Which of the following linear chain alcohols is likely to have the highest standard entropy in the liquid state?
(Multiple Choice)
4.9/5  (41)
(41)
What is the equilibrium constant for the reaction below at 298 K? 2C(s)+ 3H2(g)→ C2H6(g)
Given: ΔrH° = -84.68 kJ; ΔrS° = -173.8 J/K at 298 K.(R = 8.314 J/K⋅mol)
(Multiple Choice)
4.8/5  (36)
(36)
Thermodynamics can be used to determine all of the following except _____.
(Multiple Choice)
4.9/5  (29)
(29)
Calculate the enthalpy of vaporization of water at its normal boiling point.ΔS° [H2O( ![Calculate the enthalpy of vaporization of water at its normal boiling point.ΔS° [H<sub>2</sub>O( )] = 69.9 J/K⋅mol and ΔS° [H<sub>2</sub>O(g)] = 188.8 J/K⋅mol.](https://storage.examlex.com/TB7480/11eac9a9_4b8b_55b5_acc3_b1f6c9ae7ee2_TB7480_11.jpg) )] = 69.9 J/K⋅mol and ΔS° [H2O(g)] = 188.8 J/K⋅mol.
)] = 69.9 J/K⋅mol and ΔS° [H2O(g)] = 188.8 J/K⋅mol.
(Short Answer)
4.8/5  (32)
(32)
What is the sign of ΔH (system)and ΔS (system)if a chemical reaction is spontaneous only at lower temperatures under standard conditions?
(Multiple Choice)
4.8/5  (38)
(38)
Using the given data,determine ΔrG° at 298 K for the precipitation reaction below. Ag+(aq)+Br−(aq)→ AgBr(s)
Substance
ΔfG°(kJ/mol)at 298 K
Br−(aq)
-104.0
Ag+(aq)
77)12
AgBr(s)
-96)9
(Multiple Choice)
4.8/5  (38)
(38)
Calculate ΔrG° at 25.0 °C for the reaction below. 2 Na(s)+ 2 H2O(  )→ 2 NaOH(aq)+ H2(g)
Given: ΔrH° = −366.6 kJ/mol-rxn and ΔrS° = −154.2 J/K⋅mol-rxn.
)→ 2 NaOH(aq)+ H2(g)
Given: ΔrH° = −366.6 kJ/mol-rxn and ΔrS° = −154.2 J/K⋅mol-rxn.
(Multiple Choice)
4.9/5  (37)
(37)
A flask containing helium gas is released into a closed room.Which of the following ideas concerning entropy is/are true?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following is true of the deposition of a gaseous substance?
(Multiple Choice)
4.7/5  (38)
(38)
In any chemical process,energy must be conserved.This is the _____ law of thermodynamics.
(Short Answer)
4.9/5  (40)
(40)
Which of the following is the second law of thermodynamics?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following is correct for the condensation of gaseous oxygen at -188 °C? (The normal boiling point of oxygen is -183 °C.)
(Multiple Choice)
4.8/5  (32)
(32)
For which of the following reactions will the entropy of a system decrease?
(Multiple Choice)
4.8/5  (35)
(35)
The dissolution of ammonium nitrate occurs spontaneously in water at 25 °C.As ammonium nitrate dissolves,the temperature of the water decreases.What are the signs of ΔrH,ΔrS,and ΔrG for this process?
(Multiple Choice)
4.8/5  (42)
(42)
The standard free energy change associated with the dissolution of ammonium nitrate in water is -6.73 kJ/mol at 298.15 K. NH4NO3(s) 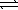 NH4NO3(aq)
Which of the following is the equilibrium constant for the reaction? (R = 8.314 J/K⋅mol)
NH4NO3(aq)
Which of the following is the equilibrium constant for the reaction? (R = 8.314 J/K⋅mol)
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)