Exam 13: The Solid State
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Palladium crystallizes in a face-centered cubic unit cell.If the edge length of the unit cell is 387 pm,what is the radius of a palladium atom in picometers?
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
A
Sodium chloride crystallizes in a(n)_____ cubic unit cell with chloride ions occupying the lattice points.The sodium ions occupy interstitial regions,with each cation in contact with six chloride ions.
Free
(Short Answer)
4.9/5  (30)
(30)
Correct Answer:
face-centered
Which of the following statements is true about semiconductors?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
Strontium oxide has a face-centered cubic unit cell of oxide ions with the strontium in octahedral holes.If the radius of Sr2+ is 127 pm and the density of SrO is 4.7 g/cm3,what is the radius of the oxide ion? (100 cm = 1 × 1012 pm)
(Multiple Choice)
4.9/5  (29)
(29)
The metal vanadium crystallizes in a body-centered cubic lattice.If the density of vanadium is 6.11 g/cm3,what is the atomic radius of vanadium?
(Multiple Choice)
4.7/5  (42)
(42)
Which equation represents the number of atoms in a body-centered cubic unit cell?
(Multiple Choice)
4.9/5  (38)
(38)
If an ionic solid has a face-centered cubic lattice of anions (Xn−)and all the octahedral holes are occupied by metal cations (Mm+),what is the formula for the compound?
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following ionic compounds is expected to have the lowest enthalpy of fusion?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following compounds is expected to have the strongest ionic bonds?
(Multiple Choice)
4.7/5  (39)
(39)
Arrange the three common cubic unit cells in order from least dense to most dense packing.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following compounds is expected to have the strongest ionic bonds?
(Multiple Choice)
4.9/5  (27)
(27)
Point D on the phase diagram is referred to as the _____ point. 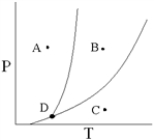
(Multiple Choice)
4.8/5  (44)
(44)
Cesium crystallizes in the body-centered cubic system.If the edge of the unit cell is 612 pm,what is the radius of a cesium atom in picometers?
(Multiple Choice)
4.8/5  (41)
(41)
Which one of the following substances is incorrectly matched with the kind of solid it forms? Substance/Kind of Solid
(Multiple Choice)
5.0/5  (30)
(30)
The bandgap of ZnTe is 218 kJ/mol.What is the maximum wavelength of light that can excite an electron transition across the band gap? (h = 6.626 × 10-34 J·s; c = 3.000 × 108 m/s)
(Multiple Choice)
4.9/5  (31)
(31)
The metal sodium crystallizes in a body-centered cubic lattice.If the density of sodium is 0.968 g/cm3,what is the unit cell edge length?
(Multiple Choice)
4.8/5  (32)
(32)
If an ionic compound with the formula MX forms a primitive cubic unit cell with the anions (Xn-)at the lattice points,the cations (M2n+)will occupy:
(Multiple Choice)
4.9/5  (35)
(35)
In metals,there are not enough electrons to fill all of the electronic energy levels.At 0 K,the highest energy level filled is referred to as the ________.
(Multiple Choice)
4.8/5  (32)
(32)
A sketch of a phase diagram is given below. 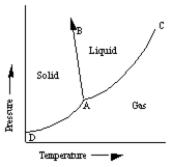 Which statement about this diagram is not true?
Which statement about this diagram is not true?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 1 - 20 of 62
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)