Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
C
Exactly 149.6 J will raise the temperature of 10.0 g of a metal from 25.0 °C to 60.0 °C.What is the specific heat capacity of the metal?
Free
(Multiple Choice)
4.9/5  (26)
(26)
Correct Answer:
A
A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.103-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
The heat required to convert a solid at its melting point to a liquid is called the heat of ________.
(Short Answer)
4.8/5  (41)
(41)
A chemical reaction in a bomb calorimeter evolves 3.86 kJ of energy in the form of heat.If the temperature of the bomb calorimeter increases by 4.17 K,what is the heat capacity of the calorimeter?
(Multiple Choice)
4.9/5  (42)
(42)
The following reaction of iron oxide with aluminum is an exothermic reaction. Fe2O3(s)+ 2 Al(s)→ 2 Fe(s)+ Al2O3(s)
The reaction of 5.00 g of Fe2O3 with excess Al(s)evolves 26.6 kJ of energy in the form of heat.Calculate the enthalpy change per mole of Fe2O3.
(Multiple Choice)
4.7/5  (36)
(36)
The thermochemical equation for the combustion of methanol is given below. CH3OH( 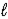 )+ 3/2 O2(g)→ CO2(g)+ 2 H 2O(g)
ΔrH° = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g of CH3OH?
)+ 3/2 O2(g)→ CO2(g)+ 2 H 2O(g)
ΔrH° = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g of CH3OH?
(Multiple Choice)
4.9/5  (39)
(39)
Determine the standard enthalpy of formation of calcium carbonate from the thermochemical equations given below. Ca(OH)2(s)→ CaO(s)+ H2O( 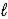 )
ΔrH° = 65.2 kJ/mol-rxn
Ca(OH)2(s)+ CO2(g)→ CaCO3(s)+ H2O(
)
ΔrH° = 65.2 kJ/mol-rxn
Ca(OH)2(s)+ CO2(g)→ CaCO3(s)+ H2O( 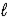 )
ΔrH° = −113.8 kJ/mol-rxn
C(s)+ O2(g)→ CO2(g)
ΔrH° = −393.5 kJ/mol-rxn
2 Ca(s)+ O2(g)→ 2 CaO(s)
ΔrH° = −1270.2 kJ/mol-rxn
)
ΔrH° = −113.8 kJ/mol-rxn
C(s)+ O2(g)→ CO2(g)
ΔrH° = −393.5 kJ/mol-rxn
2 Ca(s)+ O2(g)→ 2 CaO(s)
ΔrH° = −1270.2 kJ/mol-rxn
(Multiple Choice)
4.8/5  (37)
(37)
Which of these physical changes would require the release of energy?
(Multiple Choice)
4.8/5  (27)
(27)
44.0 g of ice at -20.0 °C is mixed with 325 g of water at 32.1 °C.Calculate the final temperature of the mixture.Assume that no energy in the form of heat is transferred to the environment.(Heat of fusion = 333 J/g; specific heat capacities: ice = 2.06 J/g⋅K,liquid water = 4.184 J/g⋅K)
(Multiple Choice)
4.7/5  (40)
(40)
Determine the heat of evaporation of carbon disulfide, CS2( 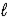 )→ CS2(g)
Given the enthalpies of reaction below.
C(s)+ 2 S(s)→ CS2(
)→ CS2(g)
Given the enthalpies of reaction below.
C(s)+ 2 S(s)→ CS2( 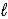 )
ΔrH° = +89.4 kJ/mol-rxn
C(s)+ 2 S(s)→ CS2(g)
ΔrH° = +116.7 kJ/mol-rxn
)
ΔrH° = +89.4 kJ/mol-rxn
C(s)+ 2 S(s)→ CS2(g)
ΔrH° = +116.7 kJ/mol-rxn
(Multiple Choice)
4.9/5  (28)
(28)
If 35.0 g H2O at 22.7 °C is combined with 65.0 g H2O at 87.5 °C,what is the final temperature of the mixture? The specific heat capacity of water is 4.184 J/g⋅K.
(Multiple Choice)
4.8/5  (28)
(28)
In a reaction,the values of ∆H and ∆U are always the same irrespective of the path taken to reach the products from reactants.
(True/False)
4.8/5  (29)
(29)
How much energy is gained by copper when 68.4 g of copper is warmed from 13.4 °C to 78.4 °C? The specific heat capacity of copper is 0.385 J/(g·°C).
(Multiple Choice)
4.9/5  (40)
(40)
If 46.1 g of copper at 11.6 °C is placed in 85.0 g of water at 72.4 °C,what is the final temperature of the mixture? The specific heat capacities of copper and water are 0.385 J/g ⋅ K and 4.184 J/g ⋅ K,respectively.
(Multiple Choice)
4.8/5  (35)
(35)
If q = 39 kJ and w = -74 kJ for a certain process,it most likely _____.
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following thermodynamic quantities are state functions: heat (q),work (w),enthalpy change (ΔH),and/or internal energy change (ΔU)?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)