Exam 2: Lets Review: The Tools of Quantitative Chemistry
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
The absolute zero point on the Kelvin scale is equal to _____.
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
A
The SI base unit of length is the ____.
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
C
Calcium carbonate,or limestone,is relatively insoluble in water.At 25 °C,only 5.8 mg will dissolve in 1.0 liter of water.What volume of water is needed to dissolve 5.0 g of calcium carbonate?
Free
(Multiple Choice)
4.7/5  (45)
(45)
Correct Answer:
E
A particular liquid boils at -374°F.What is its boiling point on the Kelvin scale?
(Multiple Choice)
4.9/5  (36)
(36)
You can identify a metal by carefully determining its density.A 29.51 g sample of an unknown metal is 1.50 cm long,2.50 cm wide,and 1.00 cm thick.What is the possible identity of the element?
(Multiple Choice)
4.8/5  (35)
(35)
Convert 1.400× 10-8 liters to nanoliters and express the answer in fixed notation using the correct number of significant figures.
(Multiple Choice)
4.8/5  (37)
(37)
Convert 0.390 ng to milligrams and express the answer in scientific notation using the correct number of significant figures.
(Multiple Choice)
4.9/5  (39)
(39)
Light with a wavelength of 1.5 × 10-8 m is in the X-ray region of the electromagnetic spectrum.What is the wavelength of this light in picometers (pm)?
(Multiple Choice)
4.8/5  (43)
(43)
A cylinder has a radius of 2.38 mm and a height of 175 mm.Calculate the volume of the cylinder in liters.(Volume = πr2h)
(Multiple Choice)
4.8/5  (31)
(31)
Using the rules of significant figures,calculate the following: 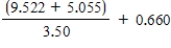
(Multiple Choice)
4.7/5  (43)
(43)
In the area of nanotechnology,particles defined as nanoparticles range in size from 1 nm to 2500 nm.One nm is equivalent to 1 × 10-9 m.If the size of the particles that make up a particular material is 5.34 × 10-8 cm,what is this size in nanometers?
(Multiple Choice)
4.7/5  (28)
(28)
What is the volume of a cube that has an edge length of 0.011 m?
(Multiple Choice)
4.8/5  (44)
(44)
How many feet (ft)make up 0.397 km if 1 mi = 1.609 km and 5280 ft = 1 mi?
(Multiple Choice)
4.9/5  (35)
(35)
A thin sheet of iridium metal that is 2.70 cm by 5.93 cm has a mass of 16.1 g and a thickness of 0.444 mm.What is the density of iridium?
(Multiple Choice)
4.9/5  (33)
(33)
A 4.75 cm3 sample of solid gallium metal has a density of 5.910 g/cm3.What volume does this sample of gallium occupy in its liquid state? The density of liquid gallium is 6.100 g/cm3.
(Multiple Choice)
4.8/5  (48)
(48)
What length of a cylindrical piece of tungsten wire having a radius of 2.27 mm has a mass of 10.0 g? The density of tungsten is 19.25 g/cm3 and Volume = πr2h.
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)