Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
What is the hybridization of the central nitrogen atom in N2O?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
For which of the following molecules and ions does the central atom have sp2 hybridization? (The central atom is listed first in each formula below.)
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
C
Which of the underlined atoms (C1,C2,N,and O)are sp2 hybridized? 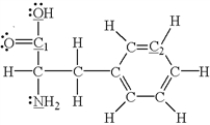
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
E
In valence bond theory,each sigma bond in CH4 is formed from the overlap of a hydrogen atom's 1s orbital with a(n)________ hybridized orbital on the carbon atom.
(Short Answer)
4.8/5  (33)
(33)
What is the molecular geometry around an atom that has 2 σ bonds,2 π bonds,and 0 lone pair of electrons?
(Multiple Choice)
4.9/5  (35)
(35)
A molecular orbital that decreases the electron density between two nuclei is said to be ____.
(Multiple Choice)
4.8/5  (45)
(45)
How many sigma and pi bonds are in the molecule pictured below? 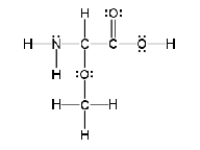
(Multiple Choice)
4.9/5  (38)
(38)
How many sigma (σ)bonds and pi (π)bonds are present in the given molecule? 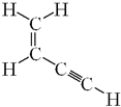
(Multiple Choice)
4.8/5  (34)
(34)
For which of the following molecules or ions does the central atom have sp hybridization: N3-,O3,and SF3+?
(Multiple Choice)
4.8/5  (26)
(26)
Refer to Diagram 9-1.According to molecular orbital theory,what is the bond order of O2-?
(Multiple Choice)
4.8/5  (38)
(38)
What is the hybridization of carbon atoms present in acetylene?
(Multiple Choice)
4.9/5  (39)
(39)
Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,which of the following species is paramagnetic?
(Multiple Choice)
4.8/5  (41)
(41)
Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
(Multiple Choice)
4.8/5  (40)
(40)
Atomic orbitals combine most effectively to form molecular orbitals when
(Multiple Choice)
4.9/5  (39)
(39)
Which theory,valence bond or molecule orbital,correctly predicts the existence of paramagnetic molecules?
(Short Answer)
4.9/5  (34)
(34)
For which of the following compounds is it possible for cis and trans isomers to exist? 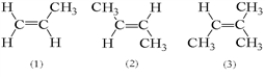
(Multiple Choice)
4.9/5  (46)
(46)
Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,determine which of the following species has the highest bond order.
(Multiple Choice)
4.9/5  (33)
(33)
Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have only one unpaired electron?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 63
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)