Exam 24: Chemistry of Coordination Compounds
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
How many iron atoms are coordinated in a hemoglobin molecule ?
(Multiple Choice)
4.8/5  (40)
(40)
Which one of the following complex ions will be paramagnetic?
(Multiple Choice)
4.9/5  (30)
(30)
The chelate effect is best attributed to considerations of which type?
(Multiple Choice)
4.9/5  (37)
(37)
What transition metal is responsible for the color of topaz _?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following cannot form both high- and low- spin octahedral complexes?
(Multiple Choice)
4.8/5  (38)
(38)
Using the following abbreviated spectrochemical series, determine which complex ion is most likely to absorb light in the red region of the visible spectrum. 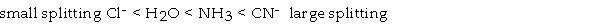
(Multiple Choice)
4.9/5  (32)
(32)
Does either or both cis- or trans- [Mn(en)2Br2] have optical isomers?
(Multiple Choice)
4.7/5  (35)
(35)
Werner's theory of primary and secondary valences for transition metal complexes has given us the concepts of and .
(Short Answer)
4.7/5  (35)
(35)
What are the donor atoms in ferrichrome and how many of them are in one molecule?
(Multiple Choice)
4.9/5  (47)
(47)
Based on electron configuration, which is most likely colorless?
(Multiple Choice)
4.8/5  (38)
(38)
Based on entropy considerations alone, which homogeneous aqueous equilibrium would be expected to lie to the right?
(Multiple Choice)
4.8/5  (37)
(37)
How can high- spin and low- spin transition metal complexes be distinguished from each other?
(Essay)
4.7/5  (35)
(35)
Showing 81 - 100 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)