Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
How many neutrons are emitted when a californium- 249 nucleus (Z=98) is bombarded with a carbon- 12 nucleus to produce a 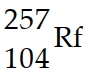 nucleus ?
nucleus ?
(Multiple Choice)
4.8/5  (32)
(32)
The initial element used to make cobalt- 60 for cancer radiation therapy is .
(Short Answer)
4.8/5  (37)
(37)
The missing product in this reaction combines with oxygen to form a compound with the formula
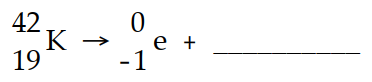
(Multiple Choice)
4.7/5  (36)
(36)
What type of reaction is known as a thermonuclear reaction?
(Multiple Choice)
4.9/5  (47)
(47)
Atoms with the same atomic number and different mass numbers
(Multiple Choice)
5.0/5  (35)
(35)
Which one of the following can be done to shorten the half- life of the radioactive decay of uranium- 238?
(Multiple Choice)
4.7/5  (25)
(25)
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 60 Ni nucleus? (The mass of a nickel- 60 nucleus is 59.9308 amu.)
(Multiple Choice)
4.8/5  (42)
(42)
41Ca decays by electron capture. The product of this reaction undergoes alpha decay. What is the product of this second decay reaction ?
(Multiple Choice)
4.9/5  (30)
(30)
Carbon- 11 decays by positron emission:
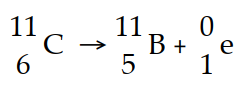 The decay occurs with a release of 2.87 × 1011 J per mole of carbon- 11. When 4.00 g of carbon- 11 undergoes this radioactive decay, _ _ g of mass is converted to energy.
The decay occurs with a release of 2.87 × 1011 J per mole of carbon- 11. When 4.00 g of carbon- 11 undergoes this radioactive decay, _ _ g of mass is converted to energy.
(Multiple Choice)
4.8/5  (35)
(35)
The beta decay of cesium- 137 has a half- life of 30 years. How many years must pass to reduce a 25 mg sample of cesium 137 to 8.7 mg?
(Multiple Choice)
4.9/5  (34)
(34)
What isotope of what element is produced if krypton- 81 undergoes beta decay?
(Short Answer)
4.9/5  (29)
(29)
The half- life for the beta decay of potassium- 40 is 1.3 × 109 years. What is the rate constant for this decay?
(Essay)
4.8/5  (32)
(32)
Electrons do not exist in the nucleus, yet beta emission is ejection of electrons from the nucleus. How does this happen?
(Essay)
4.7/5  (36)
(36)
Of the following processes, which one changes the atomic number?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)