Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
The half- life for beta decay of strontium- 90 is 28.8 years. A milk sample is found to contain 10.3 ppm strontium- 90. How many years would pass before the strontium- 90 concentration would drop to 1.0 ppm?
(Multiple Choice)
4.8/5  (31)
(31)
The amount of fissionable material necessary to maintain a chain reactions is called the
.
(Short Answer)
5.0/5  (30)
(30)
The decay of a radionuclide with a half- life of 4.3 × 105 years has a rate constant (in yr- 1) equal to
)
(Multiple Choice)
5.0/5  (36)
(36)
In the nuclear transmutation represented by 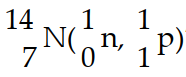 ?, what is the product?
?, what is the product?
(Multiple Choice)
4.8/5  (36)
(36)
What happens to the mass number and the atomic number of an element when it emits gamma radiation?
(Multiple Choice)
4.8/5  (35)
(35)
131I has a half- life of 8.04 days. Assuming you start with a 1.53 mg sample of 131I, how many mg will remain after 13.0 days ?
(Multiple Choice)
4.9/5  (39)
(39)
Charged particles are accelerated because the faster they move there is a greater chance of producing a nuclear reaction.
(True/False)
4.7/5  (37)
(37)
Cesium- 137 undergoes beta decay and has a half- life of 30 years. How many beta particles are emitted by a 14.0- g sample of cesium- 137 in three minutes?
(Multiple Choice)
4.9/5  (45)
(45)
The reaction shown below is responsible for creating 14C in the atmosphere. What is the bombarding particle ?
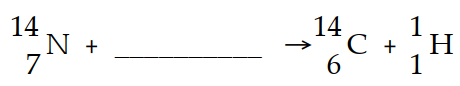
(Multiple Choice)
4.7/5  (26)
(26)
When two atoms of 2H are fused to form one atom of 4He, the total energy evolved is
3)83 × 10- 12 J. What is the total change in mass (in kg) for this reaction? (C = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (41)
(41)
The missing product in this reaction would be found in which group of the periodic table?
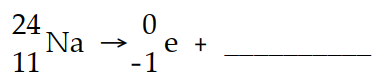
(Multiple Choice)
4.7/5  (41)
(41)
Which one of the following is used as a radiotracer to study blood?
(Multiple Choice)
4.9/5  (35)
(35)
The relative biological effectiveness (RB
E) is 10 fold greater for gamma radiation than for alpha radiation.
(True/False)
4.9/5  (38)
(38)
What happens in the nucleus of an atom that undergoes positron emission?
(Essay)
4.8/5  (36)
(36)
In balancing the nuclear reaction 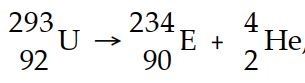 , the identity of element E is .
, the identity of element E is .
(Multiple Choice)
4.9/5  (28)
(28)
Showing 41 - 60 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)