Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Alpha decay produces a new nucleus whose than those respectively of the original nucleus.
(Multiple Choice)
4.8/5  (24)
(24)
The carbon- 14 dating method can be used to determine the age of a
(Multiple Choice)
4.8/5  (35)
(35)
Gamma radiation only changes the atomic number but not the mass number of a nucleus.
(True/False)
4.9/5  (37)
(37)
Which one of the following processes results in an increase in the atomic number?
(Multiple Choice)
4.8/5  (37)
(37)
Nuclei above the belt of stability can lower their neutron- to- proton ratio by _
(Multiple Choice)
4.9/5  (35)
(35)
Due to the nature of the positron, _ is actually detected in positron emission tomography.
(Multiple Choice)
4.9/5  (28)
(28)
In the nuclear transmutation represented by 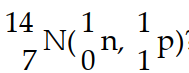 ?, what is the emitted particle?
?, what is the emitted particle?
(Multiple Choice)
4.9/5  (34)
(34)
When living tissue is irradiated most of the energy is absorbed by .
(Short Answer)
4.9/5  (45)
(45)
In the nuclear transmutation,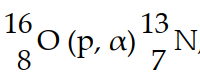 , what is the bombarding particle?
, what is the bombarding particle?
(Multiple Choice)
4.9/5  (28)
(28)
The half- life of cobalt- 60 is 5.2 yr. How many milligrams of a 2.000- mg sample remains after 6.55 years?
(Multiple Choice)
4.9/5  (32)
(32)
When an isotope undergoes electron capture, what happens to the captured electron?
(Essay)
4.7/5  (35)
(35)
Radioactive seeds that are implanted into a tumor are coated with to stop alpha and beta ray penetration.
(Short Answer)
4.9/5  (29)
(29)
In the nuclear transmutation represented by 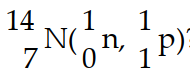 ?, what is the bombarding particle?
?, what is the bombarding particle?
(Multiple Choice)
4.8/5  (35)
(35)
Carbon- 11, fluorine- 18, oxygen- 15 and nitrogen- 13 are all used in the clinical diagnostic technique known as _ .
(Short Answer)
4.9/5  (29)
(29)
What happens to the mass number and the atomic number of an element when it undergoes beta decay?
(Multiple Choice)
4.8/5  (31)
(31)
The mass of a proton is 1.673 × 10- 24 g. The mass of a neutron is 1.675 × 10- 24 g. The mass of the nucleus of an 56Fe atom is 9.289 × 10- 23 g. What is the nuclear binding energy (in J) for 56Fe?
(c = 3.00 × 108 m/s)
(Multiple Choice)
4.8/5  (27)
(27)
Control rods in a nuclear reactor are composed of boron and .
(Short Answer)
4.8/5  (40)
(40)
Showing 81 - 100 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)