Exam 20: Electrochemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Which of the following reactions is a redox reaction?
a. K2CrO4 + BaCl2 - BaCrO4 + 2KCl
b. Pb22+ + 2Br- - PbBr
c. Cu + S - CuS
(Multiple Choice)
4.8/5  (34)
(34)
What is the coefficient of Fe3+ when the following equation is balanced? 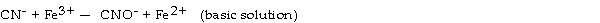
(Multiple Choice)
4.9/5  (39)
(39)
The standard cell potential (E°cell) for the reaction below is +1.10 V. The cell potential for this reaction is V when the concentration of [Cu2+] = 1.0 × 10- 5 M and [Zn2+] = 1.0 M. ![The standard cell potential (E°<sub>cell</sub>) for the reaction below is +1.10 V. The cell potential for this reaction is V when the concentration of [Cu<sup>2</sup><sup>+</sup>] = 1.0 × 10<sup>-</sup><sup> </sup><sup>5</sup><sup> </sup>M and [Zn<sup>2</sup><sup>+</sup>] = 1.0 M.](https://storage.examlex.com/TB1819/11ead62c_1f23_1803_ac95_f9aa52127bd4_TB1819_00.jpg)
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following reactions will occur spontaneously as written?
(Multiple Choice)
4.9/5  (30)
(30)
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by .
(Multiple Choice)
4.8/5  (33)
(33)
The standard emf for the cell using the overall cell reaction below is +0.48 V:
![The standard emf for the cell using the overall cell reaction below is +0.48 V: The emf generated by the cell when [Ni<sup>2</sup><sup>+</sup>] = 2.50 M and [Zn<sup>2</sup><sup>+</sup>] = 0.100 M is V.](https://storage.examlex.com/TB1819/11ead62c_1f1f_9588_ac95_77b6977504b8_TB1819_00.jpg) The emf generated by the cell when [Ni2+] = 2.50 M and [Zn2+] = 0.100 M is V.
The emf generated by the cell when [Ni2+] = 2.50 M and [Zn2+] = 0.100 M is V.
(Multiple Choice)
4.8/5  (33)
(33)
The standard reduction potential, E o
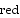 , is proportional to the stoichiometric coefficient.
, is proportional to the stoichiometric coefficient.
(True/False)
4.8/5  (37)
(37)
The balanced half- reaction in which dichromate ion is reduced to chromium metal is a process.
(Multiple Choice)
4.8/5  (44)
(44)
Showing 81 - 90 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)