Exam 20: Electrochemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
How many grams of Ca metal are produced by the electrolysis of molten CaBr2 using a current of
30)0 amp for 10.0 hours ?
(Multiple Choice)
4.9/5  (39)
(39)
The lead- containing reactant(s) consumed during recharging of a lead- acid battery is/are
)
(Multiple Choice)
4.9/5  (31)
(31)
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. 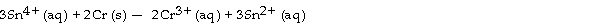
(Multiple Choice)
4.8/5  (37)
(37)
The electrolysis of molten AlCl3 for 3.25 hr with an electrical current of 15.0 A produces
G of aluminum metal.
(Multiple Choice)
4.8/5  (22)
(22)
The standard cell potential (E°cell) of the reaction below is +0.126 V. The value of OG° for the reaction is kJ/mol.
Pb (s) + 2H+ (aq) -Pb2+ (aq) + H2 (g)
(Multiple Choice)
4.9/5  (35)
(35)
Which substance is serving as the reducing agent in the following reaction? 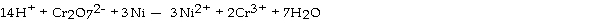
(Multiple Choice)
4.8/5  (27)
(27)
The anode of the alkaline battery is powdered zinc in a gel that contacts .
(Short Answer)
4.7/5  (29)
(29)
Consider an electrochemical cell based on the reaction:
 Which of the following actions would change the measured cell potential?
Which of the following actions would change the measured cell potential?
(Multiple Choice)
4.8/5  (38)
(38)
How many minutes will it take to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 35.2 amps in an electrolyte cell ?
(Multiple Choice)
4.9/5  (34)
(34)
Which transformation could take place at the anode of an electrochemical cell?
(Multiple Choice)
4.8/5  (34)
(34)
The half- reaction occurring at the anode in the balanced reaction shown below is . 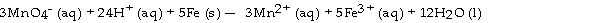
(Multiple Choice)
4.9/5  (30)
(30)
Disadvantages of the methanol fuel cell compared to the hydrogen fuel cell are consumption of catalyst and environmentally safe product.
(True/False)
4.8/5  (34)
(34)
The most useful ore of aluminum is bauxite, in which Al is present as hydrated oxides,
Al2O3 ÷ H2O. The number of kilowatt- hours of electricity required to produce 4.00 kg of aluminum from electrolysis of compounds from bauxite is when the applied emf is
5)00 V.
(Multiple Choice)
4.9/5  (40)
(40)
Using Table 20.1, which substance can be oxidized by O2 (g) in acidic aqueous solution?
(Multiple Choice)
4.7/5  (36)
(36)
When the cell potential is negative in a voltaic cell the cell reaction will not proceed spontaneously.
(True/False)
4.9/5  (35)
(35)
What current (in a) is required to plate out 1.22 g of nickel from a solution of Ni2+ in 1.0 hour __________ ?
(Multiple Choice)
4.9/5  (29)
(29)
The standard reduction potential of X is 1.23 V and that of Y is - 0.44 V therefore X is oxidized by Y.
(True/False)
4.8/5  (35)
(35)
Showing 21 - 40 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)