Exam 11: Intermolecular Forces, Liquids, and Solids
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
solids consist of atoms or molecules held together by dipole- dipole forces, London disperson forces, and/or hydrogen bonds.
(Multiple Choice)
4.9/5  (32)
(32)
As a solid element melts, the atoms become and they have _ attraction for one another.
(Multiple Choice)
4.8/5  (22)
(22)
Under ordinary conditions, a substance will sublime rather than melt if its triple point occurs at a pressure above atmospheric pressure.
(True/False)
4.7/5  (31)
(31)
The boiling points of normal hydrocarbons are higher than those of branched hydrocarbons of similar molecular weight because the London- dispersion forces between normal hydrocarbons are greater than those between branched hydrocarbons.
(True/False)
4.8/5  (34)
(34)
A substance that expands to fill its container yet has a density approaching that of a liquid, and that can behave as a solvent is called a(n) _.
(Multiple Choice)
4.9/5  (42)
(42)
The type of solid that is characterized by low melting point, softness, and low electrical conduction is a covalent- network solid.
(True/False)
4.9/5  (28)
(28)
The strongest interparticle attractions exist between particles of a and the weakest interparticle attractions exist between particles of a .
(Multiple Choice)
4.8/5  (29)
(29)
What fraction of the volume of each corner atom is actually within the volume of a face- centered cubic unit cell?
(Multiple Choice)
4.7/5  (37)
(37)
Based on molecular mass and dipole moment of the five compounds in the table below, which should have the highest boiling point? 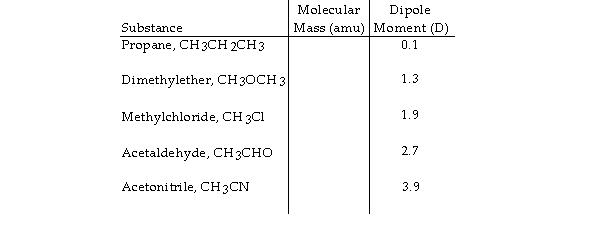
(Multiple Choice)
4.8/5  (34)
(34)
is the energy required to expand the surface area of a liquid by a unit amount of area.
(Multiple Choice)
4.8/5  (42)
(42)
The phase diagram of a substance is given above. This substance is a at 25°C and 1.0 atm.
(Multiple Choice)
4.9/5  (33)
(33)
Consider the following statements about crystalline solids:
(i) Molecules or atoms in molecular solids are held together via intermolecular forces.
(ii) Metallic solids have atoms in the points of the crystal lattice.
(iii) Ionic solids have formula units in the point of the crystal lattice.
(iv) Atoms in covalent- network solids are connected via a network of covalent bonds.
Which of the statements is false?
(Multiple Choice)
4.8/5  (28)
(28)
Crystalline solids differ from amorphous solids in that crystalline solids have .
(Multiple Choice)
5.0/5  (34)
(34)
The vapor pressure of any substance at its normal boiling point is
(Multiple Choice)
4.8/5  (31)
(31)
Together, liquids and solids constitute _ phases of matter.
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the following cannot form a solid with a lattice based on the sodium chloride structure? 
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)