Exam 10: Gases
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Given the equation 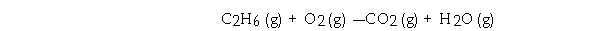 Determine the number of liters of O2 consumed at STP when 270.0 grams of C2H6 is burned.
Determine the number of liters of O2 consumed at STP when 270.0 grams of C2H6 is burned.
(Short Answer)
4.8/5  (39)
(39)
The density of ammonia gas in a 4.32 L container at 837 torr and 45.0 °C is g/L.
(Multiple Choice)
4.7/5  (30)
(30)
What volume (L) of NH3 gas at STP is produced by the complete reaction of 7.5 g of H2O according to the following reaction? 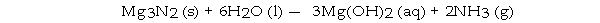
(Multiple Choice)
4.8/5  (33)
(33)
A sample of a gas originally at 25 °C and 1.00 atm pressure in a 2.5 L container is allowed to expand until the pressure is 0.85 atm and the temperature is 15 °C. The final volume of the gas is L)
(Multiple Choice)
4.8/5  (28)
(28)
A sample of oxygen gas (O2) was found to effuse at a rate equal to three times that of an unknown gas. The molecular weight of the unknown gas is g/mol.
(Multiple Choice)
4.8/5  (33)
(33)
Zinc reacts with aqueous sulfuric acid to form hydrogen gas:
 In an experiment, 225 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 748 torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is
26)74 torr.
In an experiment, 225 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 748 torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is
26)74 torr.
(Multiple Choice)
4.9/5  (42)
(42)
An ideal gas differs from a real gas in that the molecules of an ideal gas .
(Multiple Choice)
4.9/5  (28)
(28)
Arrange the following gases in order of increasing average molecular speed at 25 °C. 
(Multiple Choice)
4.7/5  (31)
(31)
The pressure in a 12.2 L vessel that contains 2.34 g of carbon dioxide, 1.73 g of sulfur dioxide, and
3)33 g of argon, all at 42 °C is mmHg.
(Multiple Choice)
4.9/5  (28)
(28)
A 1.44- g sample of an unknown pure gas occupies a volume of 0.335 L at a pressure of 1.00 atm and a temperature of 100.0 °C. The unknown gas is _.
(Multiple Choice)
4.9/5  (31)
(31)
The density of chlorine (Cl2) gas at 25 °C and 60. kPa is g/L)
(Multiple Choice)
4.7/5  (39)
(39)
A gas vessel is attached to an open- end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below.
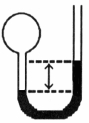 The difference in heights of the liquid in the two sides of the manometer is 32.3 cm when the atmospheric pressure is 765 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 cm when the atmospheric pressure is 765 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
(Multiple Choice)
4.9/5  (24)
(24)
A closed- end manometer was attached to a vessel containing argon. The difference in the mercury levels in the two arms of the manometer was 12.2 cm. Atmospheric pressure was 783 mmHg. The pressure of the argon in the container was mmHg.
(Multiple Choice)
4.8/5  (30)
(30)
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 667 torr. Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 7.88 L at a constant temperature of 25.0 °C.
(Multiple Choice)
4.9/5  (36)
(36)
Calcium hydride (CaH2) reacts with water to form hydrogen gas:
 How many grams of CaH2 are needed to generate 48.0 L of H2 gas at a pressure of 0.888 atm and a temperature of 32 °C?
How many grams of CaH2 are needed to generate 48.0 L of H2 gas at a pressure of 0.888 atm and a temperature of 32 °C?
(Multiple Choice)
4.9/5  (32)
(32)
The volume of 0.65 mol of an ideal gas at 365 torr and 97 °C is _ L.
(Multiple Choice)
4.9/5  (38)
(38)
The kinetic- molecular theory predicts that pressure rises as the temperature of a gas increases because _ .
(Multiple Choice)
4.8/5  (28)
(28)
Which one of the following gases would deviate the least from ideal gas behavior?
(Multiple Choice)
4.8/5  (31)
(31)
The reaction of 50 mL of Cl2 gas with 50 mL of CH4 gas via the equation:
Cl2 (g) + C2H4 (g) -C2H4Cl2 (g)
Will produce a total of mL of products if pressure and temperature are kept constant.
(Multiple Choice)
4.8/5  (28)
(28)
Showing 21 - 40 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)