Exam 10: Gases
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
A flask contains a mixture of He and Ne at a total pressure of 2.6 atm. There are 2.0 mol of He and
5)0 mol of Ne in the flask. The partial pressure of He is atm.
(Multiple Choice)
4.8/5  (34)
(34)
The pressure of a sample of CH4 gas (6.022 g) in a 30.0 L vessel at 402 K is _ atm.
(Multiple Choice)
4.8/5  (35)
(35)
A balloon originally had a volume of 4.39 L at 44 °C and a pressure of 729 torr. The balloon must be cooled to °C to reduce its volume to 3.78 L (at constant pressure)
(Multiple Choice)
4.8/5  (34)
(34)
A mixture of two gases was allowed to effuse from a container. One of the gases escaped from the container 1.43 times as fast as the other one. The two gases could have been _.
(Multiple Choice)
4.9/5  (38)
(38)
The mass of nitrogen dioxide contained in a 4.32 L vessel at 48 °C and 141600 Pa is _ _g)
(Multiple Choice)
4.8/5  (38)
(38)
If 50.75 g of a gas occupies 10.0 L at STP, 129.3 g of the gas will occupy L at STP.
(Multiple Choice)
4.8/5  (35)
(35)
A sample of He gas (2.0 mmol) effused through a pinhole in 53 s. The same amount of an unknown gas, under the same conditions, effused through the pinhole in 248 s. The molecular mass of the unknown gas is _ g/mol.
(Multiple Choice)
4.8/5  (39)
(39)
The density of chlorine gas at 1.21 atm and 34.9 °C is g/L.
(Multiple Choice)
4.7/5  (42)
(42)
A sample of a gas (5.0 mol) at 1.0 atm is expanded at constant temperature from 10 L to 15 L. The final pressure is atm.
(Multiple Choice)
4.8/5  (34)
(34)
The volume of fluorine gas required to react with 2.67 g of calcium bromide to form calcium fluoride and bromine at 41.0 °C and 4.31 atm is mL.
(Multiple Choice)
4.8/5  (35)
(35)
The volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62 °C. The gas is .
(Multiple Choice)
4.8/5  (24)
(24)
Ammonium nitrite undergoes thermal decomposition to produce only gases:
 What volume (L) of gas is produced by the decomposition of 35.0 g of NH4NO2 (s) at 525 ° C and
1)5 atm?
What volume (L) of gas is produced by the decomposition of 35.0 g of NH4NO2 (s) at 525 ° C and
1)5 atm?
(Multiple Choice)
4.8/5  (31)
(31)
A gas mixture of Ne and Ar has a total pressure of 4.00 atm and contains 16.0 mol of gas. If the partial pressure of Ne is 2.75 atm, how many moles of Ar are in the mixture?
(Multiple Choice)
4.8/5  (33)
(33)
The Mond process produces pure nickel metal via the thermal decomposition of nickel tetracarbonyl:
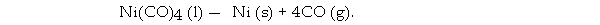 What volume (L) of CO is formed from the complete decomposition of 444 g of Ni(CO)4 at 752 torr and 22.0 °C?
What volume (L) of CO is formed from the complete decomposition of 444 g of Ni(CO)4 at 752 torr and 22.0 °C?
(Multiple Choice)
4.9/5  (20)
(20)
A gas in a 325 mL container has a pressure of 695 torr at 19 °C. There are _ _ mol of gas in the flask.
(Multiple Choice)
4.8/5  (35)
(35)
What is the partial pressure (in mm Hg) of neon in a 4.00 L vessel that contains 0.838 mol of methane, 0.184 mol of ethane, and 0.755 mol of neon at a total pressure of 928 mmHg?
(Short Answer)
4.7/5  (37)
(37)
A sample of an unknown volatile liquid was injected into a Dumas flask (mflask = 27.0928 g, Vflask = 0.1040 L) and heated until no visible traces of the liquid could be found. The flask and its contents were then rapidly cooled and reweighed (mflask+vapor = 27.4593 g). The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C, respectively. The unknown volatile liquid was .
(Multiple Choice)
4.9/5  (37)
(37)
Showing 61 - 80 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)