Exam 10: Gases
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
A sample of an ideal gas (3.00 L) in a closed container at 25.0 °C and 76.0 torr is heated to 300 °C. The pressure of the gas at this temperature is torr.
(Multiple Choice)
4.8/5  (32)
(32)
A sample of O2 gas (2.0 mmol) effused through a pinhole in 5.0 s. It will take s for the same amount of CO2 to effuse under the same conditions.
(Multiple Choice)
4.9/5  (35)
(35)
A sample of He gas (2.35 mol) occupies 57.9 L at 300.0 K and 1.00 atm. The volume of this sample is L at 423 K and 1.00 atm.
(Multiple Choice)
4.9/5  (34)
(34)
Removal of _ from the natural gas both purifies the natural gas and serves as an alternative method of production of an industrially important chemical element.
(Multiple Choice)
4.8/5  (32)
(32)
What volume (mL) of sulfur dioxide can be produced by the complete reaction of 3.82 g of calcium sulfite with excess HCl (aq), when the final SO2 pressure is 827 torr at 44.0 °C?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is not part of the kinetic- molecular theory?
(Multiple Choice)
4.9/5  (36)
(36)
Given the equation 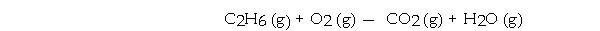 Determine the number of liters of CO2 formed at STP. when 240.0 grams of C2H6 is burned in excess oxygen gas.
Determine the number of liters of CO2 formed at STP. when 240.0 grams of C2H6 is burned in excess oxygen gas.
(Short Answer)
5.0/5  (30)
(30)
A gas at a pressure of 10.0 Pa exerts a force of N on an area of 5.5 m2.
(Multiple Choice)
4.8/5  (37)
(37)
At STP, the ratio of the root- mean- square speed of CO2 to that of SO2 is _ .
(Multiple Choice)
4.9/5  (37)
(37)
Automobile air bags use the decomposition of sodium azide as their source of gas for rapid inflation:
2NaN3 (s) - 2Na (s) + 3N2 (g).
What mass (g) of NaN3 is required to provide 40.0 L of N2 at 25.0 °C and 763 torr?
(Multiple Choice)
4.8/5  (31)
(31)
Zinc reacts with aqueous sulfuric acid to form hydrogen gas:
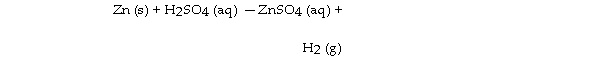 In an experiment, 201 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 733 torr. The vapor pressure of water at 27 °C is 26.74 torr. The partial pressure of hydrogen in this experiment is _ atm.
In an experiment, 201 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 733 torr. The vapor pressure of water at 27 °C is 26.74 torr. The partial pressure of hydrogen in this experiment is _ atm.
(Multiple Choice)
4.7/5  (41)
(41)
A sample of N2 gas (2.0 mmol) effused through a pinhole in 5.5 s. It will take s for the same amount of CH4 to effuse under the same conditions.
(Multiple Choice)
4.9/5  (31)
(31)
Of the following, has a slight odor of bitter almonds and is toxic.
(Multiple Choice)
4.9/5  (40)
(40)
Which noble gas is expected to show the largest deviations from the ideal gas behavior?
(Multiple Choice)
4.7/5  (32)
(32)
Arrange the following gases in order of increasing average molecular speed at 25 °C. 
(Multiple Choice)
5.0/5  (32)
(32)
Molecular compounds of low molecular weight tend to be gases at room temperature. Which of the following is most likely not a gas at room temperature?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 101 - 120 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)