Exam 40: Introduction to Quantum Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
Photoelectrons are ejected when monochromatic light shines on a freshly-prepared (oxide-free) sodium surface. In order to obtain the maximum increase in the number of electrons ejected per second, the experimenter needs to
(Multiple Choice)
4.7/5  (37)
(37)
A photon whose energy is 8.00 × 10−15 J is scattered off an electron at a 90° angle. What is the wavelength of the scattered wave?
(Multiple Choice)
4.9/5  (34)
(34)
The wavelength of a 45-kg teenager moving at 5 m/s on a skateboard is about
(Multiple Choice)
4.8/5  (37)
(37)
A neutron has a mass of 1.67 × 10−27 kg. Its de Broglie wavelength is 1.4 × 10−10 m. What is its kinetic energy (in eV)?
(Multiple Choice)
4.9/5  (41)
(41)
Your temperature is 98.6°F. Assuming your skin is a perfect radiator (ε = 1), determine the wavelength corresponding to the largest intensity (in μm).
(Multiple Choice)
4.8/5  (27)
(27)
An atomic level has a lifetime, τ, of 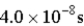 . What is the linewidth,
. What is the linewidth, 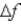 , of this level?
, of this level?
(Multiple Choice)
4.8/5  (25)
(25)
Showing 41 - 48 of 48
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)