Exam 40: Introduction to Quantum Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
The number of photons per second passing a plane perpendicular to a collimated monochromatic (one frequency) beam of light transporting power P is directly proportional to
(Multiple Choice)
4.8/5  (26)
(26)
An electron has been accelerated by a potential difference of 100 V. If its position is known to have an uncertainty of 1 nm, what is the percent uncertainty (Δp/p × 100) of the electron?
(Multiple Choice)
4.8/5  (30)
(30)
In an experiment different wavelengths of light, all able to eject photoelectrons, shine on a freshly prepared (oxide-free) zinc surface. Which statement is true?
(Multiple Choice)
4.9/5  (37)
(37)
What is the energy in eV of a photon of yellow light? λ = 500 nm.
(Short Answer)
4.7/5  (31)
(31)
The threshold wavelength for photoelectric emission of a particular substance is 500 nm. What is the work function (in eV)?
(Multiple Choice)
4.8/5  (31)
(31)
A pendulum located where 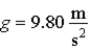 has a 0.500 kg bob and a 4.00 Hz oscillation frequency. If it is released from a point 0.150 m above the lowest point it reaches, the number of quanta in this oscillation is
has a 0.500 kg bob and a 4.00 Hz oscillation frequency. If it is released from a point 0.150 m above the lowest point it reaches, the number of quanta in this oscillation is
(Multiple Choice)
4.9/5  (42)
(42)
A photon whose wavelength is = 5.0 × 10−11 m is scattered straight backward. What is the wavelength of the scattered wave?
(Multiple Choice)
5.0/5  (43)
(43)
Assume the Heisenberg uncertainty principle can take the form ΔEΔt ≥ h/4B. How accurate can the position of an electron be made if its speed is 5 × 106 m/s and if the uncertainty in its energy is 10 eV?
(Multiple Choice)
4.8/5  (35)
(35)
An electron is accelerated through a potential difference of 25000 V. What is the de Broglie wavelength of the electron (in m)?
(Multiple Choice)
4.8/5  (31)
(31)
A baseball (1 kg) has an energy of 100 joules. If its uncertainty in position is 1 m, what is the percentage uncertainty (Δp/p × 100) in the momentum of the baseball?
(Multiple Choice)
4.7/5  (35)
(35)
Assume electrons are accelerated through a potential difference of 25000 V inside a TV picture tube. What is the minimum wavelength that could be produced when the electrons strike the phosphor? (1 Å = 10−10 m)
(Multiple Choice)
4.8/5  (31)
(31)
Because of Heisenberg's Uncertainty Principle, the velocity of a particle is known least precisely when the length of the wave packet representing the particle is
(Multiple Choice)
4.7/5  (32)
(32)
The experimental observation(s) below that require(s) a quantum explanation for the photoelectric effect
(Multiple Choice)
4.9/5  (39)
(39)
Film behind a double slit is exposed to light in the following way: First one slit is opened and light is allowed to go through that slit for time Δt. Then it is closed and the other slit is opened and light is allowed to go through that slit for the same time Δt. When the film is developed the pattern will be
(Multiple Choice)
4.9/5  (39)
(39)
Suppose we use optical radiation (λ = 500 nm) to determine the position of the electron to within the wavelength of the light. What will be the resulting uncertainty in the electron's velocity?
(Short Answer)
4.7/5  (36)
(36)
On a bright and sunny day, the intensity of solar radiation on the Earth's surface is 1 000 W/m2. If the average wavelength of the sunlight is 500 nm, how many photons are incident on a square meter of the Earth's surface per second?
(Short Answer)
4.8/5  (44)
(44)
A neutron has a mass of 1.67 × 10−27 kg. The de Broglie wavelength is 1.4 × 10−10 m. How fast is the neutron going? (in m/s)
(Multiple Choice)
4.8/5  (36)
(36)
What is the maximum velocity (in km/s) of a photoelectron emitted from a surface whose work function is 5.0 eV when illuminated by a light whose wavelength is 200 nm?
(Multiple Choice)
5.0/5  (35)
(35)
Because the factor h on the right side of the Heisenberg uncertainty principle has units of Joule-seconds, it suggests that the energy of a system also has uncertainty. The uncertainty in energy depends on the length of the time interval during which a system exists. ΔEΔt ≥ h/4π. Suppose an unstable mass is produced during a high-energy collision such that the uncertainty in its mass is me/100. (me = 9.11 × 10−31 kg.) How long will this particle exist?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 21 - 40 of 48
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)