Exam 4: Chemical Reactions
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Which species is the reducing agent in the reaction Pb(s) + 2Ag+(aq) → Pb2+(aq) + 2Ag(s)?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following is true of all combustion reactions?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is the correct set of integer coefficients for the balanced equation? ___C5H12(g) + ___O2(g) → ___CO2(g) + ___H2O(g)
(Multiple Choice)
4.9/5  (37)
(37)
0.789 mol of a particular substance weighs 142 g. What is the molar mass of this substance?
(Multiple Choice)
4.7/5  (43)
(43)
The combustion of one mole of propane, C3H8, releases 531 kcal of heat. How much heat is released by the combustion of 1.20 moles of propane?
(Multiple Choice)
4.8/5  (34)
(34)
When solutions of AgNO3 and NaOH react, the balanced molecular equation is as follows: 2AgNO3(aq) + 2NaOH(aq) → Ag2O(s) + 2NaNO3(aq) + H2O(l)
How much Ag2O is produced when 0.200 g of AgNO3 and 0.200 g of NaOH react?
(Multiple Choice)
4.8/5  (35)
(35)
Given the reaction 2HgO(s) → 2Hg(l) + O2(g), what weight of elemental mercury will be obtained by the decomposition of 0.125 mol of HgO?
(Multiple Choice)
5.0/5  (40)
(40)
Wine turns sour because of the conversion of ethanol, C2H5OH, to acetic acid, HC2H3O2. Which of the following happens to ethanol in this reaction?
(Multiple Choice)
4.9/5  (36)
(36)
In an experiment to prepare aspirin, the percent yield was 94.3%. If the actual yield was 124.3 g, what was the theoretical yield for this experiment?
(Multiple Choice)
4.9/5  (38)
(38)
Which species is oxidized in the reaction CH2O(g) + O2(g) → 2HCOOH(g)?
(Multiple Choice)
4.8/5  (39)
(39)
Which species is oxidized in the reaction Pb(s) + 2Ag+(aq) → Pb2+(aq) + 2Ag(s)?
(Multiple Choice)
4.7/5  (40)
(40)
In a blast furnace, coke, which is solid carbon, reacts with O2(g) to form carbon monoxide. The carbon monoxide then reacts with Fe2O3(s) to produce solid iron and carbon dioxide. Which of the following is the balanced equation for the reaction of carbon monoxide with Fe2O3(s)?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following can be determined using only the coefficients of the balanced chemical equation for the reaction N2(g) + 3H2(g) → 2NH3(g)?
(Multiple Choice)
4.7/5  (35)
(35)
Which species is reduced in the reaction Pb(s) + 2Ag+(aq) → Pb2+(aq) + 2Ag(s)?
(Multiple Choice)
4.7/5  (33)
(33)
How many moles of oxygen atoms are present in 5.20 mol of Al2(SO4)3?
(Multiple Choice)
4.8/5  (38)
(38)
How many moles of hydrogen atoms are there in 2.50 mol of C6H12O6?
(Multiple Choice)
5.0/5  (40)
(40)
Which species is reduced in the reaction CH2O(g) + O2(g) → 2HCOOH(g)?
(Multiple Choice)
4.9/5  (29)
(29)
The image shows several types of burners used in chemistry laboratories. 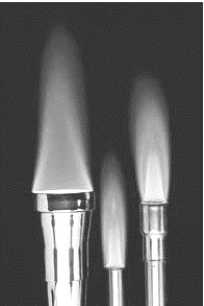 Which of the following is true irrespective of whether the burners use natural gas, propane, or butane as fuel?
Which of the following is true irrespective of whether the burners use natural gas, propane, or butane as fuel?
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following is true of all combustion reactions?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following compounds has the largest formula weight?
(Multiple Choice)
5.0/5  (40)
(40)
Showing 101 - 120 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)