Exam 4: Chemical Reactions
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
The atomic weight of copper is greater than that of chromium. Which of the following statements is true provided you have a 10-g sample of each of these metals?
(Multiple Choice)
4.8/5  (47)
(47)
0.117 mol of a particular substance weighs 21.9 g. What is the molar mass of this substance?
(Multiple Choice)
4.7/5  (42)
(42)
The atomic weight of copper is less than that of silver. Which of the following statements is true provided you have a 10-g sample of each of these metals?
(Multiple Choice)
4.8/5  (34)
(34)
Consider the following representation of a voltaic cell. 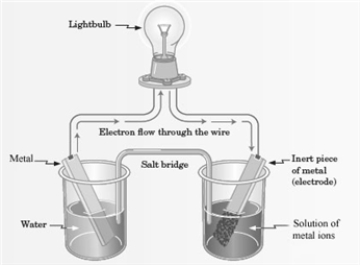 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the "Metal" shown in the diagram?
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the "Metal" shown in the diagram?
(Multiple Choice)
4.9/5  (35)
(35)
You have a sample of 1.204 × 1024 atoms of gold. How much does this sample weigh?
(Multiple Choice)
4.9/5  (36)
(36)
The temperature of an unknown substance weighing 500 grams rises by 30°C when 2,000 cal of heat is added to it. What is the specific heat of this substance?
(Multiple Choice)
4.7/5  (41)
(41)
Consider the following representation of a voltaic cell. 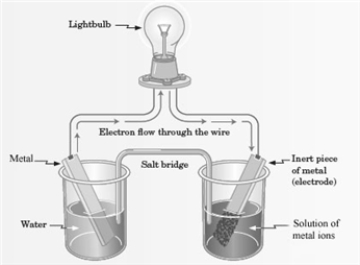 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the oxidizing agent in this reaction?
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the oxidizing agent in this reaction?
(Multiple Choice)
4.8/5  (32)
(32)
The atomic weight of platinum is less than that of gold. Which of the following statements is true provided you have a 10-g sample of each of these metals?
(Multiple Choice)
4.8/5  (37)
(37)
Examine the reaction taking place in the beaker in the image.  Which of the following is most likely occurring in the beaker?
Which of the following is most likely occurring in the beaker?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following ions always forms soluble compounds?
(Multiple Choice)
4.7/5  (29)
(29)
Ethanol is produced industrially by the acid-catalyzed reaction of ethylene with water. The balanced equation for this reaction is as follows: C2H4(g) + H2O(g) → C2H5OH(l)
If water is present in excess, how many grams of ethanol can be produced from 42.7 g of ethylene?
(Multiple Choice)
4.9/5  (44)
(44)
If you need a sample of 2.84 mol of Na2S, then how many grams of Na2S do you need?
(Multiple Choice)
4.8/5  (42)
(42)
What is the formula weight of ammonium carbonate, (NH4)2CO3?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 121 - 138 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)