Exam 4: Chemical Reactions
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
How much does a sample of 5.75 mol diethyl ether, C4H10O, weigh?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is analogous to the reactants in a chemical reaction?
(Multiple Choice)
5.0/5  (40)
(40)
A certain protein has a molar mass of 2.38 × 105 g. What is the mass of one molecule of this protein?
(Multiple Choice)
4.9/5  (44)
(44)
Consider the following representation of a voltaic cell. 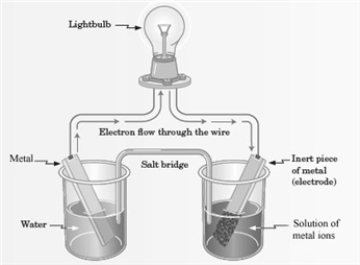 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
Which of the following is true of the Mg(s) shown in the reaction representing the voltaic cell?
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
Which of the following is true of the Mg(s) shown in the reaction representing the voltaic cell?
(Multiple Choice)
4.9/5  (35)
(35)
How many moles of marble, CaCO3, are there in a 275-g piece of marble?
(Multiple Choice)
4.9/5  (32)
(32)
The metabolism of one mole of glucose, C6H12O6, releases 6.70 × 102 kcal of heat. How much heat is released by the combustion of 50.0 g of glucose?
(Multiple Choice)
4.8/5  (36)
(36)
A substance weighing 1 kg is initially at 25°C. Heat of 11,000 cal of is added to it. What is the final temperature of the substance? The specific heat of the substance is 0.20 cal/g·°C.
(Multiple Choice)
4.8/5  (31)
(31)
The products of the reaction of phosphoric acid, H3PO4(aq), and solid magnesium hydroxide, Mg(OH)2(s), are aqueous magnesium phosphate and water. What is the balanced equation for this reaction?
(Multiple Choice)
4.8/5  (37)
(37)
The combustion of one mole of propane, C3H8, releases 531 kcal of heat. How much heat is released by the combustion of 4.40 g of propane?
(Multiple Choice)
4.9/5  (34)
(34)
Lime, CaO, is produced by the reaction CaCO3(s) → CaO(s) + CO2(g). What weight of CO2 is obtained by the decomposition of 38.7 g of CaCO3?
(Multiple Choice)
4.9/5  (46)
(46)
A typical deposit of cholesterol, C27H46O, in an artery has a mass of 3.90 mg. How many molecules of cholesterol are present in this deposit?
(Multiple Choice)
4.8/5  (32)
(32)
When solutions of H2SO4 and NaOH react, the balanced molecular equation is as follows: H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
How much Na2SO4 is obtained when 4.00 g of H2SO4 reacts with 2.00 g of NaOH?
(Multiple Choice)
4.7/5  (45)
(45)
A white precipitate of lead chloride, PbCl2, is formed when a solution of ammonium chloride, NH4Cl, is added to a solution of lead(II) nitrate, Pb(NO3)2. Which of the following is the net ionic equation for this reaction?
(Multiple Choice)
4.9/5  (39)
(39)
When solutions of AgNO3 and NaCl react, the balanced molecular equation is as follows: AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
How much AgCl is produced when 3.10 g of AgNO3 and 3.10 g of NaCl react?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 81 - 100 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)