Exam 22: Introduction to Oxygen and Carbon Dioxide in Physiology
Exam 1: Animals and Environments: Function on the Ecological Stage66 Questions
Exam 2: Molecules and Cells in Animal Physiology65 Questions
Exam 3: Genomics, Proteomics, and Related Approaches to Physiology64 Questions
Exam 4: Physiological Development and Epigenetics59 Questions
Exam 5: Transport of Solutes and Water67 Questions
Exam 6: Nutrition, Feeding, and Digestion77 Questions
Exam 7: Energy Metabolism68 Questions
Exam 8: Aerobic and Anaerobic Forms of Metabolism75 Questions
Exam 9: The Energetics of Aerobic Activity72 Questions
Exam 10: Thermal Relations84 Questions
Exam 11: Food, Energy, and Temperature at Work: The Lives of Mammals in Frigid Places76 Questions
Exam 12: Neurons59 Questions
Exam 13: Synapses58 Questions
Exam 14: Sensory Processes67 Questions
Exam 15: Nervous System Organization and Biological Clocks59 Questions
Exam 16: Endocrine and Neuroendocrine Physiology69 Questions
Exam 17: Reproduction68 Questions
Exam 19: Control of Movement71 Questions
Exam 20: Muscle78 Questions
Exam 21: Movement and Muscle at Work: Plasticity in Response to Use and Disuse67 Questions
Exam 22: Introduction to Oxygen and Carbon Dioxide in Physiology65 Questions
Exam 23: External Respiration: the Physiology of Breathing70 Questions
Exam 24: Transport of Oxygen and Carbon Dioxide in Body Fluids With an Introduction to Acid- Base Physiology68 Questions
Exam 25: Circulation72 Questions
Exam 26: Oxygen, Carbon Dioxide, and Internal Transport at Work: Diving by Marine Mammals63 Questions
Exam 27: Water and Salt Physiology: Introduction and Mechanisms72 Questions
Exam 29: Kidneys and Excretion With Notes on Nitrogen Excretion89 Questions
Exam 30: Water, Salts, and Excretion at Work: Mammals of Deserts and Dry Savannas64 Questions
Exam 28: Water and Salt Physiology of Animals in Their Environments87 Questions
Select questions type
How does diffusion work in allowing a water beetle to use an air bubble as a gill? Why does the bubble not collapse?
Free
(Essay)
5.0/5  (36)
(36)
Correct Answer:
If a water beetle grabs a fresh bubble from the atmosphere, it will use the oxygen in the bubble during its natural respiratory processes. As the partial pressure of O2 in the bubble drops, it will eventually drop below that of the surrounding water. Once this occurs, the oxygen in the water will diffuse into the air bubble, allowing it to act like a gill. The bubble does not collapse because oxygen diffuses into it and because the principal gas in the bubble, N2, tends to remain.
Which of the following contributes to a respiratory gas partial pressure in solution?
I. O2
II. CO2
III. O2 bound to hemoglobin
IV. HCO3- dissolved in plasma
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
Which statement regarding the diffusion of gases is false?
Free
(Multiple Choice)
5.0/5  (39)
(39)
Correct Answer:
D
Which statement about gases dissolved in aqueous solutions is true?
(Multiple Choice)
4.8/5  (36)
(36)
Gas transport in animals often occurs by alternation of convection and diffusion. Explain.
(Essay)
4.9/5  (31)
(31)
Which factor is a constant according to the universal gas law?
(Multiple Choice)
4.8/5  (45)
(45)
Refer to the figure shown.
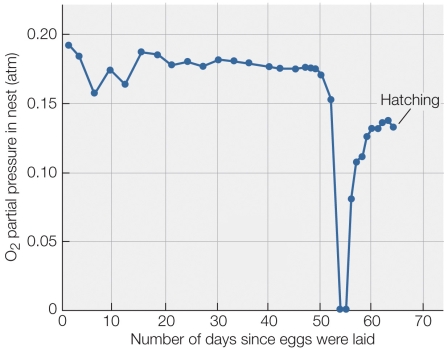 According to the figure, what was the most likely cause of the zeroing of oxygen partial pressure in the nest?
According to the figure, what was the most likely cause of the zeroing of oxygen partial pressure in the nest?
(Multiple Choice)
4.7/5  (28)
(28)
Refer to the figure shown.
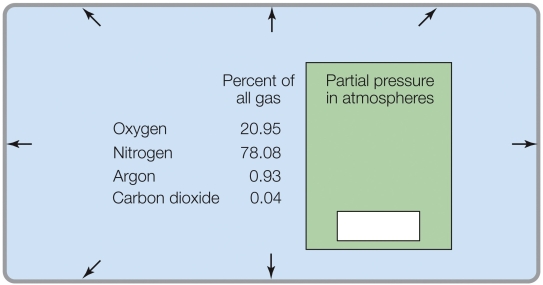 If the total pressure was 0.1 atm, the percent of O2 in the gas mixture would be _______%.
If the total pressure was 0.1 atm, the percent of O2 in the gas mixture would be _______%.
(Multiple Choice)
5.0/5  (38)
(38)
Suppose that at a temperature of 25°C and pressure of 1 atm, all the O2 is removed from 10 L of dry atmospheric air, and the remaining gas is restored to the original temperature and pressure. The final volume will be
(Multiple Choice)
4.8/5  (34)
(34)
If active oxygen transport existed, one of its advantages would be
(Multiple Choice)
4.8/5  (30)
(30)
According to the universal gas law, _______ is(are) inversely proportional to the partial pressure.
(Multiple Choice)
4.8/5  (33)
(33)
Compare and contrast the respiratory stresses of terrestrial versus aquatic environments.
(Essay)
4.7/5  (45)
(45)
In the analogy of the cascading waterfall, the height of each waterfall is analogous to
(Multiple Choice)
4.7/5  (38)
(38)
If a water beetle has been using an air bubble as a gill for 1 hour, the O2 partial pressure will be _______ that of air and the N2 partial pressure will be _______ that of air.
(Multiple Choice)
4.7/5  (33)
(33)
The fraction of the total moles of gas in a gas mixture is called the
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)