Exam 22: Introduction to Oxygen and Carbon Dioxide in Physiology
Exam 1: Animals and Environments: Function on the Ecological Stage66 Questions
Exam 2: Molecules and Cells in Animal Physiology65 Questions
Exam 3: Genomics, Proteomics, and Related Approaches to Physiology64 Questions
Exam 4: Physiological Development and Epigenetics59 Questions
Exam 5: Transport of Solutes and Water67 Questions
Exam 6: Nutrition, Feeding, and Digestion77 Questions
Exam 7: Energy Metabolism68 Questions
Exam 8: Aerobic and Anaerobic Forms of Metabolism75 Questions
Exam 9: The Energetics of Aerobic Activity72 Questions
Exam 10: Thermal Relations84 Questions
Exam 11: Food, Energy, and Temperature at Work: The Lives of Mammals in Frigid Places76 Questions
Exam 12: Neurons59 Questions
Exam 13: Synapses58 Questions
Exam 14: Sensory Processes67 Questions
Exam 15: Nervous System Organization and Biological Clocks59 Questions
Exam 16: Endocrine and Neuroendocrine Physiology69 Questions
Exam 17: Reproduction68 Questions
Exam 19: Control of Movement71 Questions
Exam 20: Muscle78 Questions
Exam 21: Movement and Muscle at Work: Plasticity in Response to Use and Disuse67 Questions
Exam 22: Introduction to Oxygen and Carbon Dioxide in Physiology65 Questions
Exam 23: External Respiration: the Physiology of Breathing70 Questions
Exam 24: Transport of Oxygen and Carbon Dioxide in Body Fluids With an Introduction to Acid- Base Physiology68 Questions
Exam 25: Circulation72 Questions
Exam 26: Oxygen, Carbon Dioxide, and Internal Transport at Work: Diving by Marine Mammals63 Questions
Exam 27: Water and Salt Physiology: Introduction and Mechanisms72 Questions
Exam 29: Kidneys and Excretion With Notes on Nitrogen Excretion89 Questions
Exam 30: Water, Salts, and Excretion at Work: Mammals of Deserts and Dry Savannas64 Questions
Exam 28: Water and Salt Physiology of Animals in Their Environments87 Questions
Select questions type
When a diving beetle is underwater with its air bubble, oxygen diffuses from the
(Multiple Choice)
4.9/5  (39)
(39)
Refer to the figure shown.
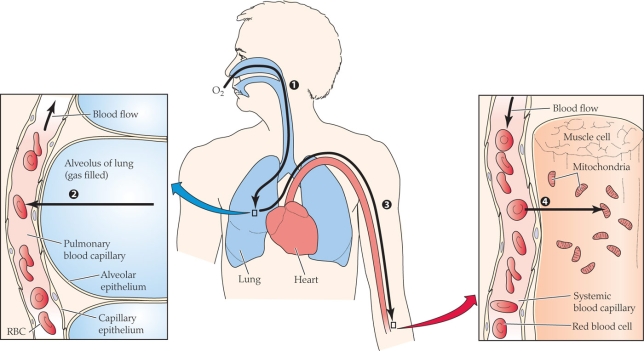 At what point(s) in the figure is the partial pressure of oxygen the lowest?
At what point(s) in the figure is the partial pressure of oxygen the lowest?
(Multiple Choice)
4.8/5  (34)
(34)
Which statement regarding gas mixtures in aqueous solutions is false?
(Multiple Choice)
4.9/5  (39)
(39)
One of the most important aspects of whether a particular microenvironment will experience hypoxia is
(Multiple Choice)
4.9/5  (43)
(43)
If 2 L of air at 0°C contains 420 mL of O2, how many mL of O2 does it contain if the air is warmed to 24°C?
(Multiple Choice)
4.8/5  (41)
(41)
Which statement regarding the diffusion of materials between gas mixtures and aqueous solutions is false?
(Multiple Choice)
4.8/5  (41)
(41)
Why are volumes of gases expressed at standard conditions of temperature and pressure (STP)?
(Multiple Choice)
4.7/5  (39)
(39)
The Krogh diffusion coefficient (K) for O2 in air is _______ K for O2 in water.
(Multiple Choice)
4.7/5  (39)
(39)
Why is it important for scuba divers to understand the concept of diffusion, especially as it pertains to N2?
(Essay)
4.8/5  (35)
(35)
Refer to the figure shown.
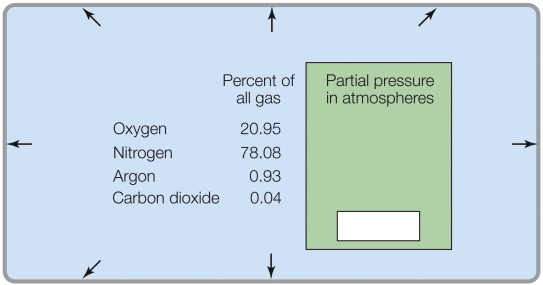 According to the data in the figure, the partial pressures at sea level total
According to the data in the figure, the partial pressures at sea level total
(Multiple Choice)
4.8/5  (35)
(35)
Which molecules are respiratory gases or chemical forms of respiratory gases?
I. O2
II. O3
III. CO2
IV. HCO3-
V. N2
(Multiple Choice)
4.8/5  (41)
(41)
Compare and contrast mole fractional concentration versus volume fractional concentration.
(Essay)
4.9/5  (39)
(39)
What are the three most important characteristics of gases dissolved in aqueous solutions? Give examples in your answer.
(Essay)
4.8/5  (38)
(38)
Refer to the figure shown.
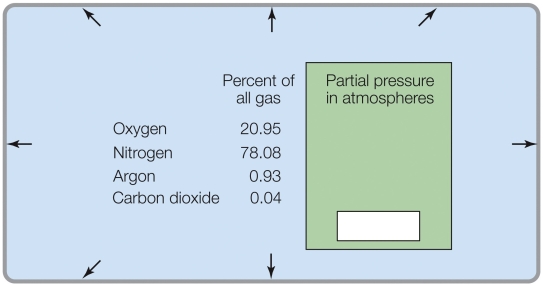 If the total pressure was 0.5 atm, the partial pressure of O2 would be _______ atm.
If the total pressure was 0.5 atm, the partial pressure of O2 would be _______ atm.
(Multiple Choice)
4.9/5  (40)
(40)
Consider two gas mixtures that are identical in temperature. The concentration of O2 in mixture #1 is 20 mmol/L, and the concentration of O2 in mixture #2 is 60 mmol/L. Which statement about the mixtures is true?
(Multiple Choice)
4.8/5  (39)
(39)
Explain the two mechanisms by which oxygen and carbon dioxide move within organisms.
(Essay)
4.8/5  (30)
(30)
The _______ of a particular gas in a mixture is the dissolved concentration of that gas when the partial pressure is 1 atm.
(Multiple Choice)
4.7/5  (30)
(30)
What is STP and why is it important when expressing volumes of gases? Include information about the relationship between STP and 1 mole of gas.
(Essay)
4.8/5  (28)
(28)
Showing 41 - 60 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)