Exam 2: Basic Chemistry
Exam 1: A View of Life49 Questions
Exam 2: Basic Chemistry57 Questions
Exam 3: The Chemistry of Organic Molecules48 Questions
Exam 4: Cell Structure and Function54 Questions
Exam 5: Membrane Structure and Function50 Questions
Exam 6: Metabolism: Energy and Enzymes55 Questions
Exam 7: Photosynthesis42 Questions
Exam 8: Cellular Respiration48 Questions
Exam 9: The Cell Cycle and Cellular Reproduction54 Questions
Exam 10: Meiosis and Sexual Reproduction54 Questions
Exam 11: Mendelian Patterns of Inheritance58 Questions
Exam 12: Molecular Biology of the Gene42 Questions
Exam 13: Regulation of Gene Expression48 Questions
Exam 14: Biotechnology and Genomics48 Questions
Exam 15: Darwin and Evolution53 Questions
Exam 16: How Populations Evolve45 Questions
Exam 17: Speciation and Macroevolution53 Questions
Exam 18: Origin and History of Life54 Questions
Exam 19: Taxonomy,systematics,and Phylogeny52 Questions
Exam 20: Viruses,bacteria,and Archaea41 Questions
Exam 21: Protist Evolution and Diversity42 Questions
Exam 22: Fungi Evolution and Diversity52 Questions
Exam 23: Plant Evolution and Diversity51 Questions
Exam 24: Flowering Plants: Structure and Organization55 Questions
Exam 25: Flowering Plants: Nutrition and Transport52 Questions
Exam 26: Flowering Plants: Control of Growth Responses54 Questions
Exam 27: Flowering Plants: Reproduction44 Questions
Exam 28: Invertebrate Evolution51 Questions
Exam 29: Vertebrate Evolution51 Questions
Exam 30: Human Evolution48 Questions
Exam 31: Animal Organization and Homeostasis48 Questions
Exam 32: Circulation and Cardiovascular Systems51 Questions
Exam 33: The Lymphatic and Immune Systems53 Questions
Exam 34: Digestive Systems and Nutrition52 Questions
Exam 35: Respiratory Systems45 Questions
Exam 36: Body Fluid Regulation and Excretory Systems47 Questions
Exam 37: Neurons and Nervous Systems49 Questions
Exam 38: Sense Organs50 Questions
Exam 39: Locomotion and Support Systems48 Questions
Exam 40: Hormones and Endocrine Systems47 Questions
Exam 41: Reproductive Systems51 Questions
Exam 42: Animal Development49 Questions
Exam 43: Behavioral Ecology48 Questions
Exam 44: Population Ecology47 Questions
Exam 45: Community and Ecosystem Ecology51 Questions
Exam 46: Major Ecosystems of the Biosphere54 Questions
Exam 47: Conservation of Biodiversity47 Questions
Select questions type
Which of the following statements is NOT true about electron configurations?
(Multiple Choice)
4.8/5  (32)
(32)
A research article indicates that researchers have used an isotope 3H to trace a certain metabolic process.From the symbol that is given,we know this is a hydrogen isotope with
(Multiple Choice)
4.8/5  (31)
(31)
Prior to prescription medications to control stomach acid and "heart burn" people consumed baking soda (sodium bicarbonate)to decrease their discomfort.This would indicate that sodium bicarbonate
(Multiple Choice)
4.7/5  (30)
(30)
The electrons are unequally shared in _______,and transferred in __________.
(Multiple Choice)
4.9/5  (25)
(25)
Draw two hydrogen atoms using Bohr's model.Now bond them to form a molecule of hydrogen gas.Write the molecular formula.Explain what type of bond you've created and why this is a stable situation.
(Not Answered)
This question doesn't have any answer yet
Classify the following substances as either hydrophobic or hydrophilic:

(Not Answered)
This question doesn't have any answer yet
Study the figures to determine which is liquid water and which is frozen water (ice).Explain your answer and predict if the water in Figure 2 would float or sink in the water in Figure 1. 
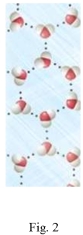
(Not Answered)
This question doesn't have any answer yet
Use Bohr's model to draw a sodium (Na)atom and a chlorine (Cl)atom.Using your model,explain what happens when sodium reacts with chlorine to form table salt.Include in your explanation ion and ionic bond formation.Use your model to help you to decide whether NaCl is hydrophilic or hydrophobic. 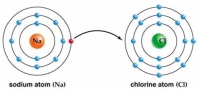
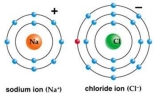
(Not Answered)
This question doesn't have any answer yet
A change of one pH unit represents a ten-fold increase or decrease in hydroxyl ion concentration.
(True/False)
4.8/5  (37)
(37)
The scale indicates the relative concentrations of hydrogen and hydroxyl ions in a solution.
(True/False)
4.9/5  (42)
(42)
Which of the following elements would be more reactive with other elements?
(Multiple Choice)
4.8/5  (25)
(25)
The characteristic way in which atoms of an element react is most related to the
(Multiple Choice)
4.7/5  (31)
(31)
 Study the chart to determine the relationship between H+ concentration and pH.If you were to create a herbal remedy to decrease excess stomach acid,would you create a solution with a relatively greater or lesser number of hydrogen ions.
Study the chart to determine the relationship between H+ concentration and pH.If you were to create a herbal remedy to decrease excess stomach acid,would you create a solution with a relatively greater or lesser number of hydrogen ions.
(Not Answered)
This question doesn't have any answer yet
Which of the following elements is NOT one of the six most common elements in living organisms?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 21 - 40 of 57
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)