Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Tell the type of decay process occurring in the following nuclear reaction. 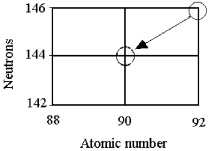
(Multiple Choice)
4.8/5  (33)
(33)
According to the law of multiple proportions,if 12 g of carbon combine with 16 g of oxygen to form CO,the number of grams of carbon that combine with 16 g of oxygen in the formation of CO2 is ________.
(Short Answer)
4.8/5  (39)
(39)
According to history,the concept that all matter is composed of atoms was first proposed by
(Multiple Choice)
4.7/5  (43)
(43)
10% saline solution (sodium chloride dissolved in water)is an example of a ________ mixture.
(Short Answer)
4.8/5  (31)
(31)
Which one of the following combinations of neutrons/protons results in the lowest number of nonradioactive (stable)isotopes?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following is the correct chemical formula for a molecule of bromine?
(Multiple Choice)
4.9/5  (35)
(35)
In which of the following sets do all species have the same number of electrons?
(Multiple Choice)
5.0/5  (40)
(40)
The element antimony has an atomic weight of 121.757 amu and only two naturally-occurring isotopes.One isotope has an abundance of 57.3% and an isotopic mass of 120.904 amu.Based on these data,what is the mass of the other isotope?
(Multiple Choice)
4.9/5  (38)
(38)
In the reaction HBr + NaOH → H2O + NaBr,If 81 g HBr react with 40 g of NaOH to produce 18 g of H2O,the number of grams of NaBr produced is ________.
(Short Answer)
4.8/5  (32)
(32)
Which of the following drawings depicts a chemical reaction consistent with Dalton's atomic theory? 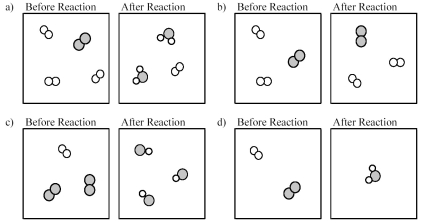
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following figures represents 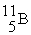 ? Unshaded spheres represent neutrons and shaded spheres represent protons.
? Unshaded spheres represent neutrons and shaded spheres represent protons. 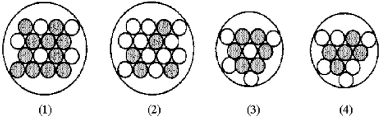
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following drawings depicts a chemical reaction consistent with Dalton's atomic theory? 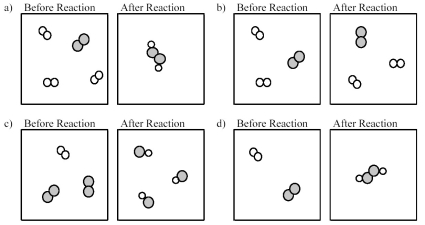
(Multiple Choice)
4.9/5  (30)
(30)
By analogy with the oxoanions of sulfur,H2TeO3 would be named
(Multiple Choice)
4.9/5  (29)
(29)
What nuclide is formed when 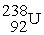 Undergoes a portion of the decay series: alpha,beta,beta,alpha,alpha,alpha.
Undergoes a portion of the decay series: alpha,beta,beta,alpha,alpha,alpha.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following nuclides is most likely to undergo beta decay?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 181 - 200 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)