Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Butyric acid has the structural formula given below. 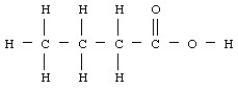 What is the molecular or chemical formula for butyric acid?
What is the molecular or chemical formula for butyric acid?
(Multiple Choice)
4.8/5  (22)
(22)
Which of the compounds,Li3P,PH3,C2H6,IBr3,are ionic compounds?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following elements would be expected to be particularly stable?
(Multiple Choice)
4.9/5  (41)
(41)
The term "nucleons" refers to the number of ________ in the atom.
(Multiple Choice)
4.8/5  (36)
(36)
A radioisotope which is neutron poor and very heavy is most likely to decay by
(Multiple Choice)
4.8/5  (34)
(34)
The charge-to-mass ratio of an electron was established by
(Multiple Choice)
4.8/5  (36)
(36)
Tell the type of decay process occurring in the following nuclear reaction. 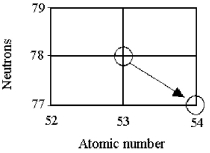
(Multiple Choice)
4.8/5  (39)
(39)
A neutral atom with atomic number 5 and mass number 11 contains ________ electrons.
(Short Answer)
4.8/5  (30)
(30)
Methane and oxygen react to form carbon dioxide and water.What mass of water is formed if 0.80 g of methane reacts with 3.2 g of oxygen to produce 2.2 g of carbon dioxide?
(Multiple Choice)
4.8/5  (39)
(39)
In which of the following sets do all species have the same number of protons?
(Multiple Choice)
4.9/5  (39)
(39)
Use the periodic table below to answer the following questions. 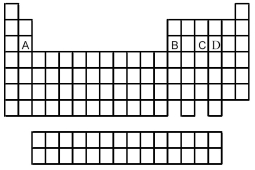 -In which pair are both formulas of binary fluorides of element C correct?
-In which pair are both formulas of binary fluorides of element C correct?
(Multiple Choice)
4.9/5  (38)
(38)
The symbol that is usually used to represent atomic number is ________.
(Multiple Choice)
4.7/5  (31)
(31)
If unshaded spheres represent sulfur atoms and shaded spheres represent oxygen atoms,which of the following drawings depicts a collection of sulfur trioxide molecules? 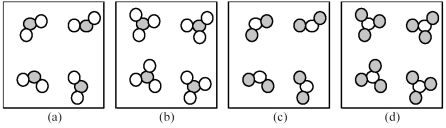
(Multiple Choice)
4.9/5  (36)
(36)
In which set do all elements tend to form cations in binary ionic compounds?
(Multiple Choice)
4.9/5  (49)
(49)
Use the periodic table below to answer the following questions. 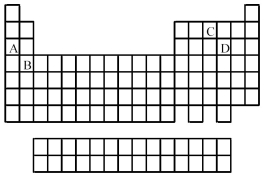 -Which elements commonly form covalent bonds?
-Which elements commonly form covalent bonds?
(Multiple Choice)
4.9/5  (41)
(41)
Which element can form more than one kind of monatomic ion?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 221 - 240 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)