Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
A radioisotope has a neutron/proton ratio which is too low.Which of the following processes will not occur for such a nucleus?
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following elements would be expected to be particularly stable?
(Multiple Choice)
4.9/5  (30)
(30)
The symbol of the isotope having Z = 88 and A = 226 is ________.
(Short Answer)
4.8/5  (46)
(46)
Which of the following nuclides is most likely to undergo beta decay?
(Multiple Choice)
4.8/5  (31)
(31)
In which of the following sets do all species have the same number of electrons?
(Multiple Choice)
4.9/5  (37)
(37)
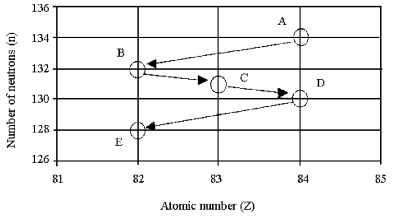 -What kind of decay process is occurring in the decay of isotope C to isotope D in the figure shown above?
-What kind of decay process is occurring in the decay of isotope C to isotope D in the figure shown above?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following nuclides is most likely to decay by electron capture?
(Multiple Choice)
4.8/5  (31)
(31)
Positron emission changes the atomic number of an element by
(Multiple Choice)
4.8/5  (39)
(39)
The number of atoms in 23 g of Na is ________ (greater than,less than,the same as)the number of atoms in 12 g of C.
(Short Answer)
4.9/5  (38)
(38)
Which of the following statements is not a postulate of Dalton's atomic theory?
(Multiple Choice)
4.9/5  (41)
(41)
The formula of iron(III)oxide contains ________ iron(III)and ________ oxide ions.
(Short Answer)
4.9/5  (40)
(40)
How many protons (p),neutrons (n),and electrons (e)are in one atom of 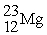 ?
?
(Multiple Choice)
4.9/5  (42)
(42)
A sample of pure lithium carbonate contains 18.8.4% lithium by mass.What is the % lithium by mass in a sample of pure lithium carbonate that has twice the mass of the first sample?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following figures represents 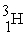 ? Unshaded spheres represent neutrons and shaded spheres represent protons.
? Unshaded spheres represent neutrons and shaded spheres represent protons. 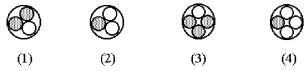
(Multiple Choice)
4.8/5  (33)
(33)
The bonding in MgO is ________,whereas the bonding in CO is ________.
(Short Answer)
4.9/5  (45)
(45)
Use the periodic table below to answer the following questions. 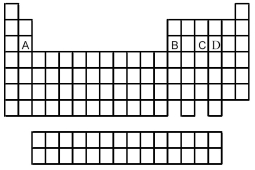 -Which is most likely to form a binary oxide with the formula MO (where M = element A,B,C,or D)?
-Which is most likely to form a binary oxide with the formula MO (where M = element A,B,C,or D)?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 21 - 40 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)