Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
In the following drawings,shaded spheres represent cations and unshaded spheres represent anions. 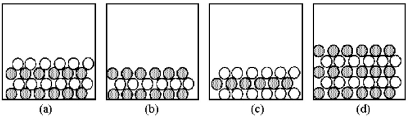 -Which drawing represents the ionic compound KNO3?
-Which drawing represents the ionic compound KNO3?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following elements would you expect to have the largest number of stable isotopes? Element number:
(Multiple Choice)
4.9/5  (41)
(41)
Most of the alpha particles directed at a thin gold foil in Rutherford's experiment
(Multiple Choice)
4.8/5  (40)
(40)
What is the chemical symbol for an atom that has 29 protons and 36 neutrons?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following figures represents 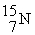 ? Unshaded spheres represent neutrons and shaded spheres represent protons.
? Unshaded spheres represent neutrons and shaded spheres represent protons. 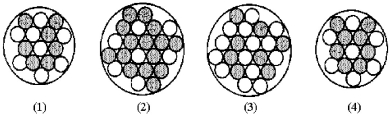
(Multiple Choice)
4.8/5  (30)
(30)
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1),which drawings (2)-(4)represent the law of multiple proportions? 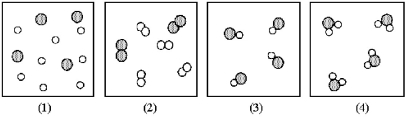
(Multiple Choice)
4.9/5  (35)
(35)
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1),which drawings (2)-(4)represent the law of multiple proportions? 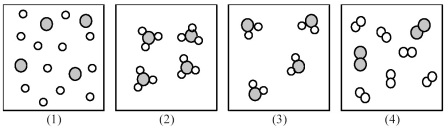
(Multiple Choice)
4.9/5  (34)
(34)
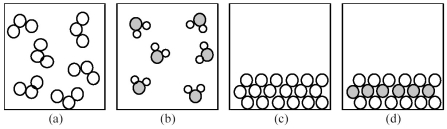 -If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents a molecular compound at room temperature and a pressure of 1 atm?
-If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents a molecular compound at room temperature and a pressure of 1 atm?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the compounds CH4,SrCl2,Cr(NO3)3,XeF2 are expected to exist as molecules?
(Multiple Choice)
4.8/5  (38)
(38)
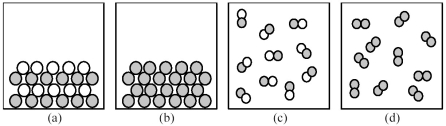 -If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
-If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
(Multiple Choice)
4.8/5  (38)
(38)
The subatomic particles contained in the nucleus of an atom are ________ and ________.
(Short Answer)
4.8/5  (32)
(32)
In a nuclear reaction,the symbol for a beta particle is ________.
(Short Answer)
4.9/5  (47)
(47)
When 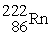 Decays in a 5-step series the product is
Decays in a 5-step series the product is 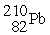 )How many alpha and beta particles are emitted in the decay series?
)How many alpha and beta particles are emitted in the decay series?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 241 - 257 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)