Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Use the periodic table below to answer the following questions. 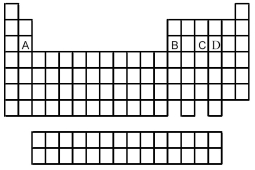 -Which is most likely to form a binary oxide with the formula M4O10 (where M = element A,B,C,or D)?
-Which is most likely to form a binary oxide with the formula M4O10 (where M = element A,B,C,or D)?
(Multiple Choice)
4.7/5  (39)
(39)
Isotopes have the same number of ________ but different numbers of ________ in their nuclei.
(Short Answer)
4.9/5  (38)
(38)
In which of the following sets do all species have the same number of protons?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the compounds,C3H8,MgCl2,Zn(NO3)2,OCl2,are expected to exist as molecules?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following is the correct chemical formula for a molecule of astatine?
(Multiple Choice)
4.8/5  (40)
(40)
The observation that 4.0 g of hydrogen reacts with 32.0 g of oxygen to form a product with
O:H mass ratio = 8:1,and 6.0 g of hydrogen reacts with 48.0 g of oxygen to form the same product
With O/H mass ratio = 8:1 is evidence for the law of
(Multiple Choice)
4.8/5  (40)
(40)
In which set do all elements tend to form cations in binary ionic compounds?
(Multiple Choice)
4.9/5  (39)
(39)
Sodium metal and water react to form hydrogen and sodium hydroxide.If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide,what mass of water was involved in the reaction?
(Multiple Choice)
4.9/5  (41)
(41)
When a substance decays by alpha radiation,the mass number of the nucleus ________ and the atomic number ________.
(Multiple Choice)
4.8/5  (38)
(38)
The atoms of a particular element all have the same number of protons as neutrons.Which of the following must be true?
(Multiple Choice)
4.8/5  (33)
(33)
Use the periodic table below to answer the following questions.  -Which is most likely to form a binary oxide with the formula MO3 (where M = element A,B,C,or D)?
-Which is most likely to form a binary oxide with the formula MO3 (where M = element A,B,C,or D)?
(Multiple Choice)
4.7/5  (36)
(36)
The missing reactant in the nuclear reaction ? → 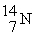 +
+ 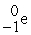 is ________.
is ________.
(Short Answer)
4.7/5  (35)
(35)
Showing 101 - 120 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)