Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1),which drawings (2)-(4)represent the law of multiple proportions? 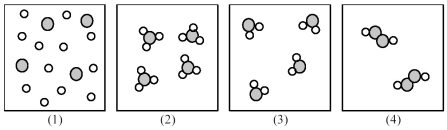
(Multiple Choice)
5.0/5  (43)
(43)
The nuclear decay process that involves the particle having the greatest mass is ________ emission.
(Multiple Choice)
4.8/5  (33)
(33)
24.0 g of which element contains the greatest number of atoms?
(Multiple Choice)
4.8/5  (37)
(37)
Use the periodic table below to answer the following questions. 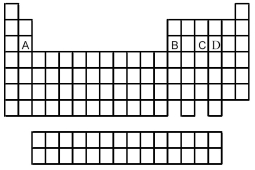 -Which is the correct formula of the binary fluoride of element B?
-Which is the correct formula of the binary fluoride of element B?
(Multiple Choice)
4.9/5  (34)
(34)
Assume that the mixture of substances in drawing (1)undergoes a chemical reaction.Which of the drawings (2)-(4)represents a product mixture that is consistent with the law of mass conservation? 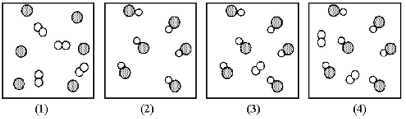
(Multiple Choice)
4.9/5  (35)
(35)
As the atomic number of the elements increases,the ratio of neutrons to protons in stable nuclei
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following represent isotopes?
[A: ![Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]](https://storage.examlex.com/TB4939/11ea7a38_c89f_9436_aa4c_0ba70ceda98c_TB4939_11.jpg) ]
[ B:
]
[ B: ![Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb47_aa4c_575c9617b6aa_TB4939_11.jpg) ]
[ C:
]
[ C: ![Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb48_aa4c_bdbe43af0171_TB4939_11.jpg) ]
[ D:
]
[ D: ![Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb49_aa4c_3385eb9c6fea_TB4939_11.jpg) ]
]
(Multiple Choice)
4.9/5  (33)
(33)
The number of atoms of carbon in 12 g of carbon is closest to .
(Multiple Choice)
4.9/5  (32)
(32)
Tell the type of decay process occurring in the following nuclear reaction. 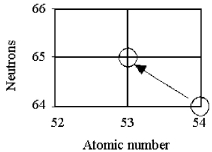
(Multiple Choice)
4.8/5  (33)
(33)
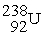 undergoes alpha decay producing one alpha particle and a single nuclide.To balance the equation,________ and ________ must be added to the right side of the equation below.
undergoes alpha decay producing one alpha particle and a single nuclide.To balance the equation,________ and ________ must be added to the right side of the equation below. 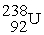 → ? + ?
→ ? + ?
(Short Answer)
4.9/5  (29)
(29)
Showing 41 - 60 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)