Exam 15: Applications of Aqueous Equilibria
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid,H2X+)are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.0500 M solution of alanine after 25.00 mL of 0.100 M NaOH has been added?
(Multiple Choice)
4.9/5  (36)
(36)
What is the resulting pH when 0.005 moles of KOH is added to 0.100 L of a buffer solution that is 0.100 M in H2PO4- and 0.100 M HPO42- and the Ka2 = 6.2 × 10-8?
(Multiple Choice)
4.9/5  (42)
(42)
What is the approximate value of the equilibrium constant,Kn,for the neutralization of nitrous acid with ammonia,shown in the equation below? The Ka for HNO2 is 4.5 × 10-4 and the Kb for NH3 is
1)8 × 10-5.
HNO2(aq)+ NH3(aq)⇌ NH4NO2(aq)
(Multiple Choice)
4.9/5  (42)
(42)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH. 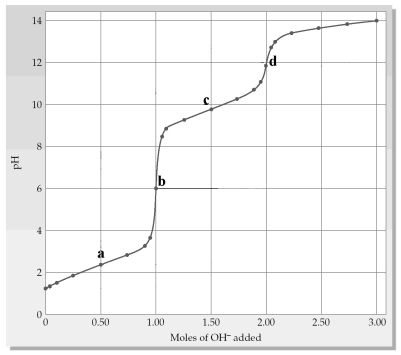 -Which point a-d represents the second equivalence point?
-Which point a-d represents the second equivalence point?
(Multiple Choice)
4.9/5  (40)
(40)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH.  -What is the pH at the first equivalence point?
-What is the pH at the first equivalence point?
(Multiple Choice)
4.8/5  (36)
(36)
What volume of 5.00 × 10-3 M HNO3 is needed to titrate 100.00 mL of 5.00 × 10-3 M Ca(OH)2 to the equivalence point?
(Multiple Choice)
4.8/5  (43)
(43)
What is the common ion in a solution prepared by mixing 0.10 M NaCH3CO2 with 0.10 M CH3CO2H?
(Multiple Choice)
4.9/5  (36)
(36)
What is the approximate pH at the equivalence point of a weak acid-strong base titration if 25 mL of aqueous formic acid requires 29.80 mL of 0.0567 M NaOH? Ka = 1.8 × 10-4 for formic acid.
(Multiple Choice)
4.9/5  (38)
(38)
What is the pH of a buffer system made by dissolving 10.70 grams of NH4Cl and 20.00 mL of 12.0 M NH3 in enough water to make 1.000 L of solution? Kb = 1.8 × 10-5 for NH3.
(Multiple Choice)
4.7/5  (44)
(44)
What is the molar solubility of CaF2 in 0.10 M NaF solution at 25°C? The Ksp for CaF2 is 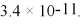
(Multiple Choice)
4.8/5  (32)
(32)
What is the approximate value of the equilibrium constant,Kn,for the neutralization of acetic acid with sodium hydroxide,shown in the equation below? The Ka for acetic acid is 1.8 × 10-5.
CH3CO2H(aq)+ NaOH(aq)⇌ H2O(l)+ NaCH3CO2(aq)
(Multiple Choice)
4.9/5  (25)
(25)
When 50 mL of 0.10 M NH4Cl is added to 50 mL of 0.10 M NH3,relative to the pH of the 0.10 M NH3 solution the pH of the resulting solution will
(Multiple Choice)
4.8/5  (36)
(36)
What is the equilibrium constant expression for the Ksp of Ca3(PO4)2?
(Multiple Choice)
4.9/5  (33)
(33)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A+ with NaOH.Which point a-d represents the isoelectric point? 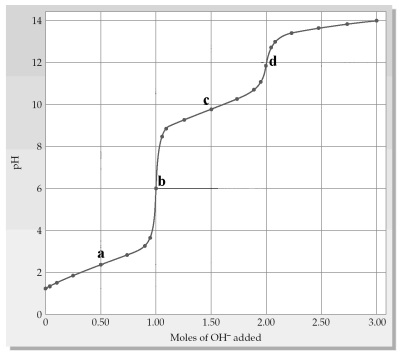
(Multiple Choice)
4.8/5  (33)
(33)
What is the chromium ion concentration for a saturated solution of Cr(OH)3 if the Ksp for Cr(OH)3 is 6.7 × 10-31?
(Multiple Choice)
4.7/5  (30)
(30)
When equal molar amounts of the following sets of compounds are mixed in water,which will not form a buffer solution?
(Multiple Choice)
4.9/5  (34)
(34)
What is the [CH3CO2-]/[CH3CO2H] ratio necessary to make a buffer solution with a pH of 4.34?
Ka = 1.8 × 10-5 for CH3CO2H.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following reactions are not consistent with the concept of acid base amphoterism?
(Multiple Choice)
4.7/5  (31)
(31)
Formic acid (HCO2H,Ka = 1.8 × 10-4)is the principal component in the venom of stinging ants.What is the molarity of a formic acid solution if 25.00 mL of the formic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 121 - 140 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)