Exam 15: Applications of Aqueous Equilibria
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Silver oxalate,Ag2C2O4,has a molar solubility = 1.1 × 10-4 mol/L.Ag2C2O4 has a solubility product Ksp = ________.
(Short Answer)
4.9/5  (39)
(39)
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid H2X+)are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.100 M solution of alanine after 100.00 mL of 0.100 M NaOH has been added?
(Multiple Choice)
4.9/5  (34)
(34)
CaF2 has Ksp = 3.5 × 10-11.If 25 mL of 8.0 × 10-4 M Ca(NO3)2 is mixed with 75 mL of
4.0 × 10-4 M KF,a precipitate of CaF2 ________ (will,will not)form.
(Short Answer)
4.9/5  (33)
(33)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH. 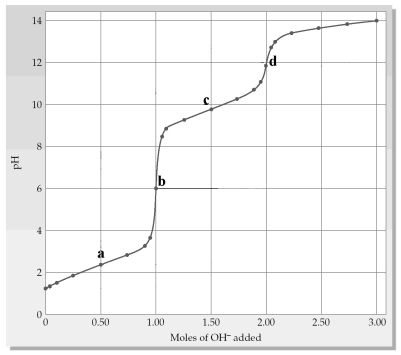 -A buffer region is indicated by which point(s)a-d?
-A buffer region is indicated by which point(s)a-d?
(Multiple Choice)
4.9/5  (36)
(36)
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH.  -Which point a-d represents pKa2?
-Which point a-d represents pKa2?
(Multiple Choice)
4.9/5  (42)
(42)
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity). 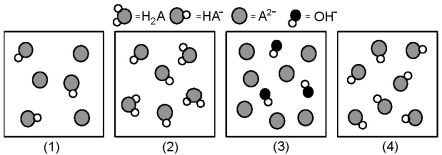 -Which picture represents the system at the first equivalence point?
-Which picture represents the system at the first equivalence point?
(Multiple Choice)
4.9/5  (43)
(43)
Which pair of ions can be separated by the addition of sulfide ion?
(Multiple Choice)
4.8/5  (35)
(35)
What is the molar solubility of AgCl in 1.0 M K2S2O3 if the complex ion Ag(S2O3)23- forms? The Ksp for AgCl is 1.8 × 10-10 and the Kf for Ag(S2O3)23- is 2.9 × 1013.
(Multiple Choice)
4.9/5  (38)
(38)
What is the pH of the resulting solution if 25.00 mL of 0.10 M acetic acid is added to 10.00 mL of 0.10 M NaOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for CH3CO2H 
(Multiple Choice)
4.9/5  (44)
(44)
A solution may contain the following ions Ag+,Cu2+,Mn2+,Ca2+,and Na+.No precipitate formed when 0.10 M NaCl was added but a dark colored precipitate formed when H2S was added to an acidic portion of the solution.After the removal of the solid the solution was made basic and more H2S was added and a dark precipitate again formed.Treatment of the filtrate with (NH4)2CO3 resulted in a white precipitate.If no further tests were made then what conclusions can you draw?
(Multiple Choice)
4.8/5  (33)
(33)
Sulfurous acid,H2SO3 has acid dissociation constants Ka1 = 1.5 × 10-2 and Ka2 = 6.3 × 10-8.What is the pH after 10.00 mL of 0.1000 M NaOH is added to 10.00 mL of 0.1000 M H2SO3?
(Multiple Choice)
4.9/5  (40)
(40)
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 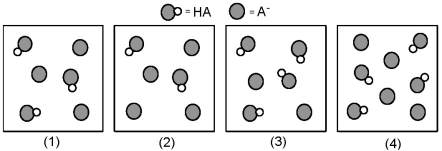 -Which solution has the largest percent dissociation of HA?
-Which solution has the largest percent dissociation of HA?
(Multiple Choice)
4.8/5  (37)
(37)
The following plot shows two titration curves,each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NaOH. 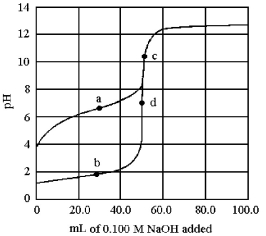 -At which point a-d is the pKa of the acid equal to the pH?
-At which point a-d is the pKa of the acid equal to the pH?
(Multiple Choice)
4.7/5  (38)
(38)
At 25°C calcium fluoride has a solubility product constant Ksp = 3.5 × 10-11.The solubility of CaF2 at this temperature is ________ mol/L.
(Short Answer)
4.9/5  (41)
(41)
What is the pH of a solution prepared by mixing 50.00 mL of 0.10 M methylamine,CH3NH2,with 20.00 mL of 0.10 M methylammonium chloride,CH3NH3Cl? Assume that the volume of the solutions are additive and that Kb = 3.70 × 10-4 for methylamine.
(Multiple Choice)
4.8/5  (40)
(40)
The neutralization constant Kn for the neutralization of phenobarbital (C12H12N2O3)and morphine (C17H19NO3)is 2.9.The acid dissociation constant Ka for phenobarbital is 3.9 × 10-8.What is the base dissociation constant Kb for morphine?
(Multiple Choice)
4.8/5  (39)
(39)
The following pictures represent solutions at various points in the titration of a weak acid HA with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity). 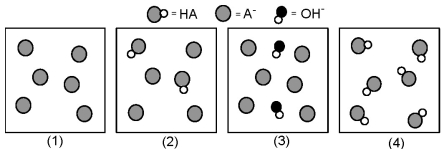 -Which picture represents the solution at the equivalence point?
-Which picture represents the solution at the equivalence point?
(Multiple Choice)
4.8/5  (35)
(35)
Which of these neutralization reactions has a pH > 7 when equal moles of acid and base are mixed?
(Multiple Choice)
5.0/5  (38)
(38)
Potassium chromate is slowly added to a solution containing 0.20 M AgNO3 and 0.20 M Ba(NO3)2.Describe what happens if the Ksp for Ag2CrO4 is 1.1 × 10-12 and the Ksp of BaCrO4 is 1.2 × 10-10.
(Multiple Choice)
4.9/5  (46)
(46)
What is the molar solubility of Mg(OH)2 in a basic solution with a pH of 12.00? Ksp for Mg(OH)2 is 5.6 × 10-12.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 161 - 180 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)