Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
How many equivalent resonance forms can be drawn for CO32-? (Carbon is the central atom.)
(Multiple Choice)
5.0/5  (40)
(40)
If more than one Lewis structure can be drawn then the molecule or ion is said to have ________ forms.
(Short Answer)
4.8/5  (30)
(30)
________ is an explosive made of nitroglycerine and an absorbent such as diatomaceous earth.
(Multiple Choice)
4.9/5  (34)
(34)
The formal charge on nitrogen in NO3- is ________,where the Lewis structure of the ion is: 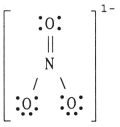
(Multiple Choice)
4.8/5  (43)
(43)
Elements from opposite sides of the periodic table tend to form ________.
(Multiple Choice)
4.8/5  (28)
(28)
Using the Born-Haber cycle,the ΔH°f of KBr is equal to ________.
(Multiple Choice)
4.8/5  (39)
(39)
For a given arrangement of ions,the lattice energy increases as ionic radius ________ and as ionic charge ________.
(Multiple Choice)
4.7/5  (38)
(38)
Dynamite consists of nitroglycerine mixed with diatomaceous earth or cellulose.What is another name for dynamite?
(Multiple Choice)
4.8/5  (34)
(34)
The only noble gas without eight valence electrons is ________.
(Multiple Choice)
4.7/5  (36)
(36)
A valid Lewis structure of ________ cannot be drawn without violating the octet rule.
(Multiple Choice)
4.8/5  (37)
(37)
Why don't we draw double bonds between the Be atom and the Cl atoms in BeCl2?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following does not have eight valence electrons?
(Multiple Choice)
5.0/5  (33)
(33)
How many equivalent resonance forms can be drawn for the nitrate ion?
(Multiple Choice)
4.8/5  (33)
(33)
For ________ forms of a molecule or ion,the observed structure is an average of the ________ forms.
(Multiple Choice)
4.8/5  (38)
(38)
Using the table of average bond energies below,the ΔH for the reaction is ________ kJ. 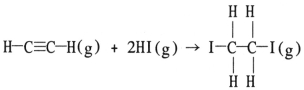 Bond: C≡C C-C H-I C-I C-H D (kJ/mol): 839 348 299 240 413
Bond: C≡C C-C H-I C-I C-H D (kJ/mol): 839 348 299 240 413
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)