Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Polyatomic ions with an even number of electrons will follow the ________ rule.
(Short Answer)
4.7/5  (34)
(34)
A nonpolar bond will form between two ________ atoms of ________ electronegativity.
(Multiple Choice)
4.8/5  (29)
(29)
There are ________ paired and ________ unpaired electrons in the Lewis symbol for a fluorine atom.
(Multiple Choice)
4.9/5  (42)
(42)
There are ________ unpaired electrons in the Lewis symbol for an oxygen atom .
(Multiple Choice)
4.8/5  (41)
(41)
The electron configuration of the sulfide ion (S2-)is ________.
(Multiple Choice)
4.8/5  (38)
(38)
How many different types of resonance structures can be drawn for the ion SO32-?
(Multiple Choice)
4.8/5  (28)
(28)
The Lewis structure of PF3 shows that the central phosphorus atom has ________ nonbonding and ________ bonding electron pair(s).
(Multiple Choice)
4.9/5  (25)
(25)
In the resonance form of ozone shown below,the formal charge on the central oxygen atom is ________. 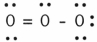
(Multiple Choice)
4.7/5  (30)
(30)
In the Lewis symbol for a nitrogen atom,there are ________ paired and ________ unpaired electrons.
(Multiple Choice)
4.9/5  (39)
(39)
The ________ ion is represented by the electron configuration [Ar]3d2.
(Multiple Choice)
4.9/5  (29)
(29)
The strength of a ________ bond is measured by its bond enthalpy.
(Short Answer)
4.7/5  (41)
(41)
Which of the following noble gas electron configurations represents the Ru+ cation?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 21 - 40 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)