Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Using the table of bond dissociation energies,the ΔH for the reverse of following gas-phase reaction is ________ kJ. 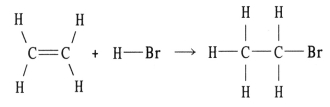
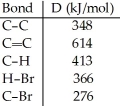
(Multiple Choice)
5.0/5  (30)
(30)
To convert from one resonance structure to another,________.
(Multiple Choice)
4.8/5  (31)
(31)
Based on the octet rule,aluminum most likely forms an ________ ion.
(Multiple Choice)
4.8/5  (54)
(54)
In some molecules and polyatomic ions,the sum of the valence electrons is odd and as a result the octet rule fails.
(True/False)
4.9/5  (38)
(38)
Of the possible bonds between carbon atoms (single,double,and triple),________.
(Multiple Choice)
4.8/5  (27)
(27)
There can be two equivalent best resonance structures of ________.
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following does not have eight valence electrons?
(Multiple Choice)
5.0/5  (36)
(36)
The oxide of which of the following metals should have the greatest lattice energy?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following species does the noble gas electron configuration [Kr]4d10 represent?
(Multiple Choice)
4.8/5  (34)
(34)
The electron configuration of the phosphide ion (P3-)is ________.
(Multiple Choice)
4.8/5  (43)
(43)
Electron affinity is a measure of how strongly an atom can attract additional electrons.
(True/False)
4.9/5  (37)
(37)
How many single covalent bonds must a chlorine atom form to have a complete octet in its valence shell?
(Multiple Choice)
4.9/5  (30)
(30)
Most explosives are compounds that decompose rapidly to produce ________ products and a great deal of ________.
(Multiple Choice)
4.8/5  (39)
(39)
Electronegativity ________ from left to right within a period and ________ from top to bottom within a group.
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the bond energy of C-F given that the heat of atomization of CHFClBr is 1502 kJ/mol,and that the bond energies of C-H,C-Br,and C-Cl are 413,276,and 328 kJ/mol,respectively.
(Essay)
4.9/5  (38)
(38)
The type of compound that is most likely to contain a covalent bond is ________.
(Multiple Choice)
4.8/5  (32)
(32)
Which energy change corresponds to the electron affinity of fluorine?
(Multiple Choice)
4.8/5  (30)
(30)
In the Lewis structure of ClF,the formal charge on Cl is ________,and the formal charge on F is ________.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 101 - 120 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)