Exam 11: Liquids and Intermolecular Forces
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Under ordinary conditions,a substance will sublime rather than melt if its triple point occurs at a pressure above atmospheric pressure.
(True/False)
4.8/5  (35)
(35)
When the phase diagram for a substance has a solid-liquid phase boundary line that has a ________ slope,the substance can go from solid to liquid,within a small temperature range,via the application of pressure.
(Multiple Choice)
4.9/5  (35)
(35)
The critical temperature and pressure of CS2 are 279 °C and 78 atm,respectively.A supercritical fluid can only exist at a temperature ________ 279 °C and pressure ________ 78 atm.
(Multiple Choice)
4.8/5  (30)
(30)
Large intermolecular forces in a substance are manifested by ________.
(Multiple Choice)
4.9/5  (37)
(37)
The phase diagram of a substance is shown above.The area labeled ________ indicates the gas phase for the substance.
(Multiple Choice)
4.9/5  (29)
(29)
For a given substance that exhibits liquid-crystalline properties,the transition from solid to liquid-crystal state occurs ________.
(Multiple Choice)
4.9/5  (37)
(37)
Which species has London dispersion forces as the only intermolecular force?
(Multiple Choice)
4.9/5  (41)
(41)
Ethanol melts at -114 °C and boils at 78 °C at a constant pressure of 1 atm.What state of matter must a sample of ethanol be in at 0°C and 1 atm?
(Multiple Choice)
4.9/5  (38)
(38)
On the phase diagram shown above,the coordinates of point B corresponds to the ________.
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the following substances will have hydrogen bonding as one of its intermolecular forces?
(Multiple Choice)
4.9/5  (38)
(38)
Ethanol ( 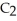
 OH)melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ)is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
OH)melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ)is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
(Multiple Choice)
5.0/5  (32)
(32)
As a gaseous element condenses,the atoms become ________ and they have ________ attraction for one another.
(Multiple Choice)
4.9/5  (28)
(28)
Based on molecular mass and dipole moment of the five compounds in the table below,which should have the highest boiling point? 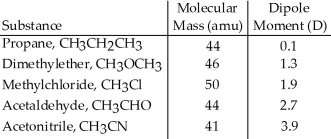
(Multiple Choice)
4.8/5  (32)
(32)
In general,the vapor pressure of a substance increases as ________ increases.
(Multiple Choice)
4.9/5  (30)
(30)
Showing 21 - 40 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)