Exam 16: Acid-Base Equilibria
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The 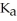 of hypochlorous acid (HClO)is 3.0 ×
of hypochlorous acid (HClO)is 3.0 × 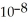 at 25.0 °C.Calculate the pH of a
at 25.0 °C.Calculate the pH of a 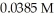 hypochlorous acid solution.
hypochlorous acid solution.
(Multiple Choice)
4.7/5  (41)
(41)
The hydride ion, 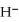 ,is a stronger base than the hydroxide ion,O
,is a stronger base than the hydroxide ion,O 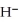 .The product(s)of the reaction of hydride ion with water is/are ________.
.The product(s)of the reaction of hydride ion with water is/are ________.
(Multiple Choice)
4.8/5  (23)
(23)
Using the data in the table,which of the conjugate bases below is the weakest base? 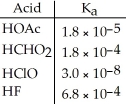
(Multiple Choice)
4.9/5  (30)
(30)
Ka for arsenic acic,HAsO42-,is 7.5 × 10-12.What is the pH of a 0.15 M aqueous solution of AsO43-?
(Multiple Choice)
4.9/5  (35)
(35)
The 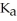 of citric acid is 1.9 ×
of citric acid is 1.9 × 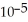 at 25.0 °C.What is the pH of a 0.35 M aqueous solution of citric acid?
at 25.0 °C.What is the pH of a 0.35 M aqueous solution of citric acid?
(Multiple Choice)
4.9/5  (35)
(35)
What is the pOH of a 0.030 M solution of calcium hydroxide?
(Multiple Choice)
4.8/5  (28)
(28)
The acid-dissociation constants of sulfurous acid (H2SO3)are Ka1 = 1.7 × 10-2 and 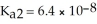 at 25.0 °C.Calculate the pH of a 0.163 M aqueous solution of sulfurous acid.
at 25.0 °C.Calculate the pH of a 0.163 M aqueous solution of sulfurous acid.
(Multiple Choice)
4.7/5  (35)
(35)
An aqueous basic solution has a concentration of 0.050 M and Kb is 4.4 × 10-4.What is the concentration of hydronium ion in this solution (M)?
(Multiple Choice)
4.9/5  (40)
(40)
An aqueous solution contains 0.390 M HCl at 25.0 °C.The pH of the solution is ________.
(Multiple Choice)
4.9/5  (28)
(28)
Using the data in the table,which of the conjugate bases below is the weakest base? 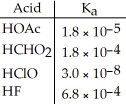
(Multiple Choice)
4.8/5  (27)
(27)
Hydrochloric acid is a strong acid.This means that ________.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following 0.5 M aqueous salt solutions will have a pH of 7.0 at 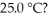 LiF RbBr NaClO4 NH4Cl
LiF RbBr NaClO4 NH4Cl
(Multiple Choice)
4.9/5  (33)
(33)
The pH of an aqueous solution at 25.0 °C is 10.55.What is the molarity of 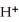 in this solution?
in this solution?
(Multiple Choice)
4.8/5  (30)
(30)
The 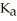 for acid HA is
for acid HA is 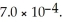 What is the pH of a 0.15 M aqueous solution of KA?
What is the pH of a 0.15 M aqueous solution of KA?
(Multiple Choice)
4.8/5  (46)
(46)
The Ka for HCN is 4.9 × 10-10.What is the value of Kb for CN-?
(Multiple Choice)
4.8/5  (25)
(25)
Showing 61 - 80 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)