Exam 20: Electrochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The balanced half-reaction in which sulfate ion is reduced to sulfite ion is a ________ process.
(Multiple Choice)
4.8/5  (34)
(34)
When the cell potential is negative in a voltaic cell,the cell reaction will not proceed spontaneously.
(True/False)
4.9/5  (32)
(32)
How many kilowatt-hours of electricity are used to produce 4.50 kg of magnesium in the electrolysis of molten 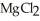 with an applied emf of 5.00 V?
with an applied emf of 5.00 V?
(Multiple Choice)
4.8/5  (38)
(38)
The electrode at which oxidation occurs is called the ________.
(Multiple Choice)
4.8/5  (36)
(36)
What is the oxidation number of nitrogen in the NH2OH molecule?
(Multiple Choice)
4.7/5  (36)
(36)
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. Cr (s)+ 3Fe3+ (aq)→ 3Fe2+ (aq)+ Cr3+ (aq)
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following reactions is a redox reaction?
(a)K2CrO4 + BaCl2 → BaCrO4 + 2KCl
(b)Pb22+ + 2Br- → PbBr
(c)Cu + S → CuS
(Multiple Choice)
4.8/5  (35)
(35)
The purpose of the salt bridge in an electrochemical cell is to ________.
(Multiple Choice)
4.9/5  (34)
(34)
Which element is reduced in the following reaction?
Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
(Multiple Choice)
5.0/5  (28)
(28)
The potential (E)to move K+ from the extracellular fluid to the intracellular fluid necessitates work.The sign for this potential is ________.
(Short Answer)
4.8/5  (37)
(37)
The less ________ the value of E°red,the lower the driving force for reduction.
(Multiple Choice)
4.8/5  (37)
(37)
The town of Natrium,West Virginia,derives its name from the sodium produced in the electrolysis of molten sodium chloride (NaCl)mined from ancient salt deposits.The number of kilowatt-hours of electricity required to produce 4.50 kg of metallic sodium from the electrolysis of molten NaCl(s)is ________ when the applied emf is 5.00 V.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 101 - 113 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)