Exam 20: Electrochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The ________ of the alkaline battery is powdered zinc in a gel that contacts potassium hydroxide.
(Short Answer)
4.9/5  (30)
(30)
________ electrons appear in the following half-reaction when it is balanced. S4O62- → 2S2O32-
(Multiple Choice)
4.7/5  (37)
(37)
The standard reduction potential,E°red,is proportional to the stoichiometric coefficient.
(True/False)
4.9/5  (34)
(34)
________ is the oxidizing agent in the reaction below. Na(s)+ 2Cl2(g)→ 2NaCl(s)
(Multiple Choice)
4.7/5  (35)
(35)
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by ________.
(Multiple Choice)
4.9/5  (31)
(31)
The balanced half-reaction in which dichromate ion is reduced to chromium metal is a ________ process.
(Multiple Choice)
4.9/5  (36)
(36)
Which element is oxidized in the reaction below?
I- + MnO4- + H+ → I2 + MnO2 + H2O
(Multiple Choice)
4.8/5  (38)
(38)
How many grams of Ca metal are produced by the electrolysis of molten Ca 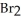 using a current of 30.0 amp for 8.0 hours?
using a current of 30.0 amp for 8.0 hours?
(Multiple Choice)
4.9/5  (39)
(39)
The amount of a substance that is reduced or oxidized is inversely proportional to the number of electrons produced in a cells half reaction.
(True/False)
4.7/5  (35)
(35)
Consider an electrochemical cell based on the reaction: 2H+ (aq)+ Sn (s)→ Sn2+ (aq)+ H2 (g)
Which of the following actions would change the measured cell potential?
(Multiple Choice)
4.9/5  (32)
(32)
What is the coefficient of the dichromate ion when the following equation is balanced?
Fe2+ + Cr2O72- → Fe3+ + Cr3+ (acidic solution)
(Multiple Choice)
4.9/5  (23)
(23)
Which substance is serving as the oxidizing agent in the following reaction?
CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(g)
(Multiple Choice)
4.9/5  (29)
(29)
How many grams of Cu are obtained by passing a current of 12 A through a solution of 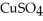 for 15 minutes?
for 15 minutes?
(Multiple Choice)
4.7/5  (33)
(33)
The most useful ore of aluminum is bauxite,in which Al is present as hydrated oxides, 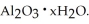 The number of kilowatt-hours of electricity required to produce
The number of kilowatt-hours of electricity required to produce 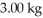 of aluminum from electrolysis of compounds from bauxite is ________ when the applied emf is
of aluminum from electrolysis of compounds from bauxite is ________ when the applied emf is 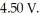
(Multiple Choice)
4.7/5  (32)
(32)
The electrolysis of molten 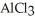 for 2.50 hr with an electrical current of 12.0 A produces
for 2.50 hr with an electrical current of 12.0 A produces  of aluminum metal.
of aluminum metal.
(Multiple Choice)
4.9/5  (28)
(28)
The balanced half-reaction in which chlorine gas is reduced to the aqueous chloride ion is a ________ process.
(Multiple Choice)
4.8/5  (43)
(43)
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. Sn2+ (aq)+ 2Fe3+ (aq)→ 2Fe2+ (aq)+ Sn4+ (aq)
(Multiple Choice)
4.8/5  (40)
(40)
Showing 81 - 100 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)