Exam 22: Chemistry of the Nonmetals
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Tetraboric acid,H2B4O7,is prepared by heating boric acid,H3BO3 (a condensation reaction involving water loss).If 400.0 mmol H3BO3 are used,what mass (g)of H2O is formed,assuming quantitative stoichiometric conversion?
(Multiple Choice)
4.9/5  (27)
(27)
Hydrogen can form hydride ions.Elements in group ________ typically form ions with the same charge as the hydride ion.
(Multiple Choice)
4.9/5  (46)
(46)
Consider the following xenon compounds:
(i)XeCl2
(ii)XeCl4
(iii)XeO4
(iv)XeOCl4
(v)XeO3
Which of the compounds is(are)polar?
(Multiple Choice)
4.8/5  (34)
(34)
Which form of elemental sulfur is the most stable at room temperature?
(Multiple Choice)
4.8/5  (42)
(42)
The oxidation numbers of sulfur in the sulfate ion,sulfite ion,sulfur trioxide,and hydrogen sulfide are ________,________,________,and ________,respectively.
(Multiple Choice)
4.8/5  (29)
(29)
Which noble gas is known to form a variety of binary compounds?
(Multiple Choice)
4.7/5  (32)
(32)
What is the coefficient of NO2 when the following disproportionation reaction is balanced?
NO2 (g)+ H2O (l)→ 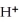 (aq)+ NO3- (aq)+ NO (g)
(aq)+ NO3- (aq)+ NO (g)
(Multiple Choice)
4.9/5  (44)
(44)
The principal combustion products of compounds containing carbon and hydrogen in the presence of excess O2 are ________.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 60 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)