Exam 22: Chemistry of the Nonmetals
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The oxidation state of chlorine in the ClO- molecule is ________.
(Multiple Choice)
4.8/5  (34)
(34)
In the following chemical equation PCl3 + 3H2O →
The products (when the equation is balanced)are ________.
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following compounds would produce the most acidic aqueous solution?
(Multiple Choice)
4.8/5  (50)
(50)
________ is formed when wood is heated strongly in the absence of air.
(Multiple Choice)
4.8/5  (32)
(32)
Of the atoms below,________ is the most effective in forming π bonds.
(Multiple Choice)
5.0/5  (37)
(37)
An aqueous solution of HF is considered a relatively ________ acid.
(Short Answer)
4.9/5  (39)
(39)
Which of the following equations correctly represents the reaction of 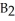
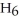 with oxygen?
with oxygen?
(Multiple Choice)
4.8/5  (39)
(39)
What method is used to produce the most hydrogen gas in the United States?
(Multiple Choice)
4.8/5  (39)
(39)
The electrical conductivity of ________ is low in the dark,but increases on exposure to light.
(Short Answer)
4.9/5  (40)
(40)
The danger from mixing ammonia with bleach is the production of ________.
(Short Answer)
5.0/5  (28)
(28)
The heavier noble gases are more reactive than the lighter ones because ________.
(Multiple Choice)
4.8/5  (45)
(45)
The primary commercial use of elemental nitrogen is in the manufacture of ________.
(Multiple Choice)
4.7/5  (37)
(37)
Which element in group 7A is not capable of having an expanded valence shell?
(Multiple Choice)
4.9/5  (33)
(33)
In a hypohalous acid,the oxidation state of the halogen is ________.
(Short Answer)
4.7/5  (38)
(38)
Showing 121 - 140 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)