Exam 22: Chemistry of the Nonmetals
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The most common isotope of hydrogen is sometimes referred to as ________.
(Multiple Choice)
4.8/5  (30)
(30)
An example of a form of pure carbon that contains only sp3 hybridized carbon atoms is ________.
(Multiple Choice)
4.9/5  (33)
(33)
The arrangement of oxygen atoms around a silicon atom in SiO44- is ________.
(Multiple Choice)
4.9/5  (36)
(36)
Of the following,which is most likely to form interstitial carbides?
(Multiple Choice)
4.8/5  (32)
(32)
In a proton transfer reaction the weaker a Br∅nsted-Lowry acid the ________ is its conjugate.
(Short Answer)
4.7/5  (41)
(41)
Sodium borohydride,NaB 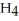 ,is a strong reducing agent because ________.
,is a strong reducing agent because ________.
(Multiple Choice)
4.9/5  (32)
(32)
Group 8A elements are all gases at room temperature except for ________.
(Short Answer)
4.8/5  (34)
(34)
The Ostwald process is by which NH3 is converted commercially into ________.
(Multiple Choice)
4.8/5  (36)
(36)
The acid and salts of which halogen-oxyanion are the most stable?
(Short Answer)
4.8/5  (30)
(30)
The oxidation state of fluorine in its compounds is ________.
(Multiple Choice)
4.9/5  (34)
(34)
Which halogen can react with fluorine to form the compound XF7?
(Multiple Choice)
4.9/5  (26)
(26)
Briefly explain why carbon and silicon can form oxides with such different physical properties,gaseous CO2 and solid SiO2.
(Essay)
4.9/5  (42)
(42)
Showing 61 - 80 of 194
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)