Exam 20: Principles of Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
All of the following statements concerning voltaic cells are true EXCEPT
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following reactions would require the use of an inert electrode when used in a voltaic cell?
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the cell potential,at 25 C,based upon the overall reaction
Zn2+(aq)+ 2 Fe2+(aq) Zn(s)+ 2 Fe3+(aq)
If [Zn2+] = 1.50 10-4 M,[Fe3+] = 0.0200 M,and [Fe2+] = 0.0100 M.The standard reduction potentials are as follows:
Zn2+(aq)+ 2 e- Zn(s) E = -0.763 V
Fe3+(aq)+ e- Fe2+(aq) E = +0.771 V
(Multiple Choice)
4.9/5  (30)
(30)
How many moles of electrons are produced from a current of 14.4 A in 3.20 hours?
(Multiple Choice)
4.9/5  (34)
(34)
The following has a potential of 0.92 V: 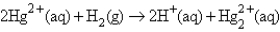 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction 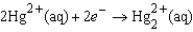 Would be
Would be
(Multiple Choice)
4.8/5  (41)
(41)
The standard cell potential of the following electrochemical cell is 0.19 V.Pt | Sn4+(aq,1.0 M),Sn2+(aq,1.0 M)|| Cu2+(aq,0.200 M)| Cu
Which factor will increase the measured cell potential of the galvanic cell?
(Multiple Choice)
4.9/5  (33)
(33)
Write a balanced half-reaction for the reduction of CrO42-(aq)to Cr(OH)3(s)in a basic solution.
(Multiple Choice)
4.9/5  (28)
(28)
When the following oxidation-reduction reaction in acidic solution is balanced,what is the lowest whole-number coefficient for K+(aq)?
K(s)+ Ca2+(aq) K+(aq)+ Ca(s)
(Multiple Choice)
4.9/5  (39)
(39)
Use the standard reduction potentials below to determine which element or ion is the best oxidizing agent.
O2(g)+ 4 H+(aq)+ 4 e- 2 H2O(  ) E = +1.229 V
Hg22+(aq)+ 2 e- 2 Hg(
) E = +1.229 V
Hg22+(aq)+ 2 e- 2 Hg(  ) E = +0.789 V
I2(s)+ 2 e- 2 I-(aq) E = +0.535 V
) E = +0.789 V
I2(s)+ 2 e- 2 I-(aq) E = +0.535 V
(Multiple Choice)
4.8/5  (28)
(28)
Given: 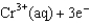
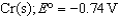
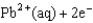
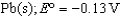 What is the standard Gibbs free-energy change for the following reaction?
What is the standard Gibbs free-energy change for the following reaction? 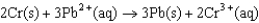
(Multiple Choice)
4.7/5  (39)
(39)
Write a balanced chemical equation for the following reaction in an acidic solution.Cr2O72-(aq)+ Ni(s) Cr3+(aq)+ Ni2+(aq)
(Multiple Choice)
4.8/5  (39)
(39)
What is the equilibrium constant (K)at 25°C for the following cell reaction? 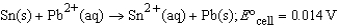
(Multiple Choice)
4.8/5  (40)
(40)
Assuming the following reaction proceeds in the forward direction,
3 Sn4+(aq)+ 2 Cr(s) 3 Sn2+(aq)+ 2 Cr3+(aq)
(Multiple Choice)
4.7/5  (51)
(51)
Which of the following equations is a correct form of the Nernst equation?
(Multiple Choice)
4.8/5  (33)
(33)
Write a balanced half-reaction for the reduction of hydrogen peroxide to water in an acidic solution.
(Multiple Choice)
4.9/5  (28)
(28)
The following electrochemical cell has a potential of +0.326 V at 25 C.Pt | H2(g,1.00 atm)| H+(aq,1.00 M)|| Cl-(aq)| AgCl(s)| Ag
The standard reduction potential,E ,of AgCl(s)= +0.222 V.What is the Cl-(aq)concentration?
(Multiple Choice)
4.8/5  (34)
(34)
Given the following standard reduction potentials,
Pb2+(aq)+ 2 e- Pb(s) E = -0.126 V
PbSO4(s)+ 2 e- Pb(s)+ SO42-(aq) E = -0.355 V
Determine Ksp for PbSO4(s)at 25 C.
(Multiple Choice)
4.8/5  (31)
(31)
A current of 15.0 A is passed through molten magnesium chloride for 15.0 h.How many moles of magnesium metal could be produced via this electrolysis?
(Multiple Choice)
4.8/5  (34)
(34)
If rG for the following reaction is -22.2 kJ/mol-rxn,calculate 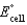 .
Cu2+(aq)+ 2 Ag(s)+ 2 Cl-(aq) Cu(s)+ 2 AgCl(s)
.
Cu2+(aq)+ 2 Ag(s)+ 2 Cl-(aq) Cu(s)+ 2 AgCl(s)
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following statements concerning a voltaic cell is/are correct?
1)Reduction occurs at the cathode.
2)A spontaneous reaction generates an electric current in a voltaic cell.
3)Without a salt bridge charge buildup will cause the cell reaction to stop.
(Multiple Choice)
4.9/5  (39)
(39)
Showing 61 - 80 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)