Exam 20: Principles of Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Calculate the equilibrium constant for the reaction below at 25 C,
Co(s)+ 2 Cr3+(aq) Co2+(aq)+ 2 Cr2+(aq)
Given the following thermodynamic information.
Co2+(aq)+ 2 e- Co(s) E = -0.28 V
Cr3+(aq)+ e- Cr2+(aq) E = -0.41 V
(Multiple Choice)
4.9/5  (37)
(37)
The fuel cells used aboard NASA's Space Shuttles are electrochemical cells that generate electricity from which overall chemical reaction?
(Multiple Choice)
5.0/5  (43)
(43)
Write a balanced chemical equation for the following reaction in a basic solution.
ClO-(aq)+ Cr(OH)3(s) Cl-(aq)+ CrO42-(aq)
(Multiple Choice)
4.8/5  (40)
(40)
Assuming the following reaction proceeds in the forward direction,
Fe3+(aq)+ Co(s) Fe2+(aq)+ Co2+(aq)
(Multiple Choice)
4.9/5  (38)
(38)
Calculate rG for the disproportionation reaction of Cu+ at 25 C,
2 Cu+(aq) Cu2+(aq)+ Cu(s)
Given the following thermodynamic information.
Cu+(aq)+ e- Cu(s) E = +0.518 V
Cu2+(aq)+ 2 e- Cu(s) E = +0.337 V
(Multiple Choice)
4.8/5  (27)
(27)
The following reaction occurs spontaneously.
2 H+(aq)+ Ca(s) Ca2+(aq)+ H2(g)
Write the balanced oxidation half-reaction.
(Multiple Choice)
5.0/5  (37)
(37)
When an aqueous solution of sodium sulfate is electrolyzed,what are the expected products?
Reduction Half-Reaction E° (V)
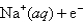 Na(s) -2.71
2H2O(l)+ 2e- H2(g)+ 2OH-(aq) -0.83
2H+(aq)+ 2e- H2(g) 0.00
O2(g)+ 4H+(aq)+ 4e- 2H2O(l) 1.23
S2O82-(aq)+ 2e- 2SO42-(aq) 2.01
Na(s) -2.71
2H2O(l)+ 2e- H2(g)+ 2OH-(aq) -0.83
2H+(aq)+ 2e- H2(g) 0.00
O2(g)+ 4H+(aq)+ 4e- 2H2O(l) 1.23
S2O82-(aq)+ 2e- 2SO42-(aq) 2.01
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements is true concerning the electrochemical cell below,
Ba(s)| Ba2+(aq,1.0 M)|| Mn2+(aq,1.0 M)| Mn(s)
And given the following standard reduction potentials?
Ba2+(aq)+ 2e- Ba(s); E° = -2.91 V
Mn2+(aq)+ 2e- Mn(s); E° = -1.19 V
(Multiple Choice)
4.9/5  (33)
(33)
Write a balanced chemical equation for the oxidation of Cd(s)by concentrated nitric acid,producing NO2(g)and Cd2+(aq).
(Multiple Choice)
4.8/5  (45)
(45)
One kind of battery used in watches contains mercury(II)oxide.As current flows,the mercury(II)oxide is reduced to mercury.
HgO(s)+ H2O( 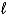 )+ 2 e- Hg(
)+ 2 e- Hg( 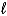 )+ 2 OH-(aq)
If 2.3 10-5 amperes flows continuously for 1200 days,what mass of Hg(
)+ 2 OH-(aq)
If 2.3 10-5 amperes flows continuously for 1200 days,what mass of Hg( 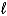 )is produced?
)is produced?
(Multiple Choice)
4.8/5  (40)
(40)
The electrochemical reaction which powers a lead-acid storage battery is as follows:
Pb(s)+ PbO2(s)+ 4H+(aq)+ 2SO42-(aq) 2PbSO4(s)+ 2H2O(l)
A single cell of this battery consists of a Pb electrode and a PbO2 electrode,each submerged in sulfuric acid.What reaction occurs at the cathode during discharge?
(Multiple Choice)
4.8/5  (42)
(42)
In an electrolytic cell,reduction occurs at the ________ and oxidation occurs at the ________.
(Short Answer)
5.0/5  (35)
(35)
Balance the following half-reaction occurring in acidic solution.
NO3-(aq) HNO2(aq)
(Multiple Choice)
4.8/5  (43)
(43)
Consider the following half-reactions:
Ag+(aq)+ e- Ag(s) E = +0.80 V
Cu2+(aq)+ 2 e- Cu(s) E = +0.34 V
Pb2+(aq)+ 2 e- Pb(s) E = -0.13 V
Fe2+(aq)+ 2 e- Fe(s) E = -0.44 V
Al3+(aq)+ 3 e- Al(s) E = -1.66 V
Which of the above metals or metal ions will oxidize Pb(s)?
(Multiple Choice)
4.8/5  (37)
(37)
Write a balanced chemical equation for the overall reaction represented by the cell notation below.
Al(s)| Al3+(aq)|| Br-(aq)| Br2(g)| Pt(s)
(Multiple Choice)
4.7/5  (38)
(38)
Given:
Mn2+(aq)+ 2e- Mn(s); E° = -1.18 V
Cu2+(aq)+ 2e- Cu(s); E° = 0.34 V
Cr2O72-(aq)+ 14H+(aq)+ 6e- 2Cr3+(aq)+ 7H2O(l); E° = 1.33 V
Which of the following species is the strongest reducing agent?
(Multiple Choice)
4.8/5  (40)
(40)
Balance the following oxidation-reduction occurring in acidic solution.
MnO4-(aq)+ Cr2+(aq) Mn2+(aq)+ Cr3+(aq)
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following statements is true concerning the voltaic cell shown below? 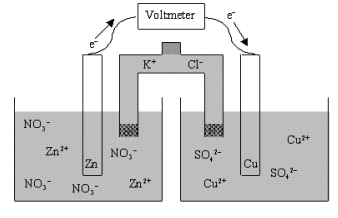
(Multiple Choice)
4.7/5  (40)
(40)
Calculate the equilibrium constant for the following reaction at 25 C,
2 IO3-(aq)+ 5 Hg( 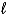 )+ 12 H+(aq) I2(s)+ 5 Hg2+(aq)+ 6 H2O(
)+ 12 H+(aq) I2(s)+ 5 Hg2+(aq)+ 6 H2O( 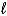 )
Given the following thermodynamic information.
IO3-(aq)+ 6 H+(aq)+ 5 e- I2(s)+ 3 H2O(
)
Given the following thermodynamic information.
IO3-(aq)+ 6 H+(aq)+ 5 e- I2(s)+ 3 H2O( 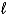 ) E = +1.20 V
Hg2+(aq)+ 2 e- Hg(
) E = +1.20 V
Hg2+(aq)+ 2 e- Hg( 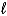 ) E = +0.86 V
) E = +0.86 V
(Multiple Choice)
4.8/5  (35)
(35)
Balance the following half-reaction occurring in basic solution.
MnO2(s) Mn(OH)2(s)
(Multiple Choice)
4.8/5  (33)
(33)
Showing 21 - 40 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)