Exam 6: The Three-Dimensional Structure of Proteins
Exam 1: The Scope of Biochemistry16 Questions
Exam 2: The Matrix of Life: Weak Interactions in an Aqueous Environment24 Questions
Exam 3: The Energetics of Life24 Questions
Exam 4: Nucleic Acids27 Questions
Exam 5: Introduction to Proteins: the Primary Level of Protein Structure24 Questions
Exam 6: The Three-Dimensional Structure of Proteins23 Questions
Exam 7: Protein Function and Evolution26 Questions
Exam 8: Contractile Proteins and Molecular Motors18 Questions
Exam 9: Carbohydrates: Sugars,saccharides,glycans27 Questions
Exam 10: Lipids,membranes and Cellular Transport24 Questions
Exam 11: Enzymes: Biological Catalysts23 Questions
Exam 12: Chemical Logic of Metabolism24 Questions
Exam 13: Carbohydrate Metabolism: Glycolysis, gluconeogenesis, glycogen Metabolism, and the Pentose Phosphate Pathway40 Questions
Exam 14: Citric Acid Cycle and Glyoxylate Cycle24 Questions
Exam 15: Electron Transport, oxidative Phosphorylation, and Oxygen Metabolism24 Questions
Exam 16: Photosynthesis25 Questions
Exam 17: Lipid Metabolism I: Fatty Acids,triacylglycerols,and Lipoproteins25 Questions
Exam 18: Interorgan and Intracellular Coordination of Energy Metabolism in Vertebrates21 Questions
Exam 19: Lipid Metabolism Ii: Membrane Lipids, steroids, isoprenoids, and Eicosanoids24 Questions
Exam 20: Metabolism of Nitrogenous Compounds I: Principles of Biosynthesis, utilization, and Turnover24 Questions
Exam 21: Metabolism of Nitogenous Compounds Ii: Amino Acids, porphyrins, and Neurotransmitters24 Questions
Exam 22: Nucleotide Metabolism24 Questions
Exam 23: Mechanisms of Signal Transduction23 Questions
Exam 24: Genes,genomes and Chromosomes24 Questions
Exam 25: Dna Replication24 Questions
Exam 26: Dna Restructuring: Repair,recombination,rearrangement,amplification24 Questions
Exam 27: Information Readout: Transcription and Post-Transcriptional Processing24 Questions
Exam 28: Information Decoding: Translation and Post-Translational Protein Processing27 Questions
Exam 29: Regulation of Gene Expression24 Questions
Select questions type
Which of the following is considered a dead-end complex (and therefore dangerous due to its resistance to proteolytic cleavage)in the protein-folding pathway?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
D
Which of the following structural proteins is correctly paired with the modified amino acid or cross-link that is an integral part of that protein?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
A
Which of the following is a highly compact structure that is very commonly used to transition from one region of secondary structure to another in a globular protein?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
The presence of a hydrophobic amino acid at every third or fourth residue in -keratin results in which of the following?
(Multiple Choice)
4.7/5  (29)
(29)
What type of interaction occurs between the -sheets of fibroin?
(Multiple Choice)
4.8/5  (36)
(36)
Show the reaction catalyzed by prolyl isomerase.Indicate the configuration of both substrate and product. 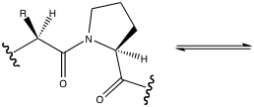
(Essay)
4.7/5  (36)
(36)
In a typical Ramachandran plot,_____ is plotted vs _____ to illustrate _______.
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following could contribute to quaternary structure?
(Multiple Choice)
5.0/5  (31)
(31)
Show the most likely interaction that would occur between a serine residue and a glutamine residue.
(Essay)
4.7/5  (29)
(29)
Draw a section of antiparallel -sheet showing two sheets with three residues in each sheet.Clearly show the hydrogen bond pattern between the two sheets.
(Essay)
4.9/5  (28)
(28)
Which of the following causes denaturation of a protein when disulfide bonds are present?
(Multiple Choice)
4.9/5  (29)
(29)
Of the following proteins that aid in the folding process,which is exclusively involved in the interconversion of cis and trans bonds?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following types of secondary structure has a non-integer value of residues per turn?
(Multiple Choice)
4.8/5  (34)
(34)
A protein with five disulfide bonds was treated with -mercaptoethanol and urea.Once the protein was denatured,the -mercaptoethanol and urea were removed by dialysis.What is the likelihood that all five disulfide bonds will reform correctly?
(Multiple Choice)
4.8/5  (33)
(33)
Which amino acid is often referred to as a "helix-breaker" due to its absence from -helices but is often found in structures such as -turns?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following contributes to a positive G for protein folding?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is true regarding -sheet structures?
(Multiple Choice)
4.9/5  (23)
(23)
If a peptide was composed entirely of -helical structure and found to contain an integer number of complete turns,which of the following would be a possible number of amino acid residues in the peptide?
(Multiple Choice)
4.9/5  (35)
(35)
The two amino acids most often found in a polyproline II helix are proline and _______.
(Multiple Choice)
4.7/5  (25)
(25)
Showing 1 - 20 of 23
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)