Exam 15: Electron Transport, oxidative Phosphorylation, and Oxygen Metabolism
Exam 1: The Scope of Biochemistry16 Questions
Exam 2: The Matrix of Life: Weak Interactions in an Aqueous Environment24 Questions
Exam 3: The Energetics of Life24 Questions
Exam 4: Nucleic Acids27 Questions
Exam 5: Introduction to Proteins: the Primary Level of Protein Structure24 Questions
Exam 6: The Three-Dimensional Structure of Proteins23 Questions
Exam 7: Protein Function and Evolution26 Questions
Exam 8: Contractile Proteins and Molecular Motors18 Questions
Exam 9: Carbohydrates: Sugars,saccharides,glycans27 Questions
Exam 10: Lipids,membranes and Cellular Transport24 Questions
Exam 11: Enzymes: Biological Catalysts23 Questions
Exam 12: Chemical Logic of Metabolism24 Questions
Exam 13: Carbohydrate Metabolism: Glycolysis, gluconeogenesis, glycogen Metabolism, and the Pentose Phosphate Pathway40 Questions
Exam 14: Citric Acid Cycle and Glyoxylate Cycle24 Questions
Exam 15: Electron Transport, oxidative Phosphorylation, and Oxygen Metabolism24 Questions
Exam 16: Photosynthesis25 Questions
Exam 17: Lipid Metabolism I: Fatty Acids,triacylglycerols,and Lipoproteins25 Questions
Exam 18: Interorgan and Intracellular Coordination of Energy Metabolism in Vertebrates21 Questions
Exam 19: Lipid Metabolism Ii: Membrane Lipids, steroids, isoprenoids, and Eicosanoids24 Questions
Exam 20: Metabolism of Nitrogenous Compounds I: Principles of Biosynthesis, utilization, and Turnover24 Questions
Exam 21: Metabolism of Nitogenous Compounds Ii: Amino Acids, porphyrins, and Neurotransmitters24 Questions
Exam 22: Nucleotide Metabolism24 Questions
Exam 23: Mechanisms of Signal Transduction23 Questions
Exam 24: Genes,genomes and Chromosomes24 Questions
Exam 25: Dna Replication24 Questions
Exam 26: Dna Restructuring: Repair,recombination,rearrangement,amplification24 Questions
Exam 27: Information Readout: Transcription and Post-Transcriptional Processing24 Questions
Exam 28: Information Decoding: Translation and Post-Translational Protein Processing27 Questions
Exam 29: Regulation of Gene Expression24 Questions
Select questions type
__________ increases oxygen consumption while decreasing ATP synthesis,but ____________ decreases both oxygen consumption and ATP synthesis.
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
D
The inhibition by an unknown inhibitor of electron transport was found to be removed by a large concentration of phenazine methosulfate.Since phenazine methosulfate is known to accept electrons from cytochrome b of complex III,which of the following is most similar to the unknown inhibitor?
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
B
Within the electron transport chain,complex _____ represents the entry point for electrons from NADH while complex _____ represents the entry point for electrons from FADH2.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
C
In the malate/aspartate shuttle,glutamate is converted to _________ in the mitochondria while aspartate is converted to ___________ in the cytosol.
(Multiple Choice)
4.8/5  (27)
(27)
What is the approximate P/O ratio if the source of electrons for the electron transport chain is FADH2?
(Multiple Choice)
4.9/5  (37)
(37)
Which amino acid is complexed to iron-sulfur clusters to enable them to associate with proteins?
(Multiple Choice)
4.7/5  (29)
(29)
The presence of 10 copies of the c subunit of the F0 complex of yeast ATP synthase allows for the synthesis of 1 ATP for every ______ proton(s)that are translocated across the inner mitochondrial membrane.
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following steps in the catalytic cycle of the F1 component is the most energetically unfavorable?
(Multiple Choice)
4.9/5  (39)
(39)
In addition to iron,what metal is found in certain cytochromes?
(Multiple Choice)
4.8/5  (31)
(31)
A(n)_______ incorporates oxygen from O2 into its products while a(n)_______ uses another oxidizing agent to accomplish the oxidation of its substrate.
(Multiple Choice)
4.7/5  (36)
(36)
Using the structure of coenzyme Q,show one reaction to produce the semiquinone and the subsequent reaction to produce the hydroquinone forms of the molecule.(R represents the isoprenoid tail). 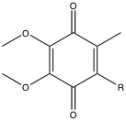
(Essay)
4.8/5  (40)
(40)
If the following two reactions were coupled,what would be the oxidizing agent for the spontaneous reaction?
NAD+ + H+ + 2e-  NADH E ' = -0.32 V
Acetaldehyde + 2H+ + 2e-
NADH E ' = -0.32 V
Acetaldehyde + 2H+ + 2e-
 ethanol E ' = -0.20 V
ethanol E ' = -0.20 V
(Multiple Choice)
4.8/5  (39)
(39)
Use the following half reactions to determine the reaction found in the pyruvate dehydrogenase complex.Calculate the G 'for the reaction.
lipoate + 2H+ + 2e-  dihydrolipoate E ' = -0.29 V
FAD + 2H+ + 2e-
dihydrolipoate E ' = -0.29 V
FAD + 2H+ + 2e-  FADH2 E ' = -0.22 V
FADH2 E ' = -0.22 V
(Essay)
4.9/5  (36)
(36)
Rotation of the ____ subunit causes the _____ subunit to change from tight conformation where ATP is produced to open conformation where ATP is released.
(Multiple Choice)
4.7/5  (47)
(47)
Which of the following correctly indicates the number of protons pumped into the intermembrane space at each complex of electron transport for each FADH2 that is oxidized by the electron transport pathway?
(Multiple Choice)
4.8/5  (32)
(32)
What is the approximate free energy for transporting a mole of protons from the matrix to the intermembrane space under normal conditions within the mitochondria?
(Multiple Choice)
4.9/5  (29)
(29)
The oxidized form of coenzyme Q is considered a _______ while the reduced form is considered a _______.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following describes a correct and complete electron transfer pathway?
(Multiple Choice)
4.7/5  (27)
(27)
Based on the following information,what is the G ' for the oxidation of malate by NAD+?
NAD+ + H+ + 2e-  NADH E '= -0.32 V
Oxaloacetate + 2H+ + 2e-
NADH E '= -0.32 V
Oxaloacetate + 2H+ + 2e-  malate E ' = -0.17 V
malate E ' = -0.17 V
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following acts as an uncoupler of the mitochondrial proton gradient due to its action as an ionophore?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 24
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)