Exam 7: Quantum Theory and Atomic Structure
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
The FM station KDUL broadcasts music at 99.1 MHz. Find the wavelength of these waves.
(Multiple Choice)
4.9/5  (42)
(42)
Consider the following adjectives used to describe types of spectrum: continuous line atomic emission absorption
How many of them are appropriate to describe the spectrum of radiation absorbed by a sample of mercury vapor?
(Multiple Choice)
4.7/5  (35)
(35)
Who developed an empirical equation from which the wavelengths of lines in the spectrum of hydrogen atoms can be calculated?
(Multiple Choice)
4.8/5  (41)
(41)
Select the arrangement of electromagnetic radiation which starts with the lowest energy and increases to greatest energy.
(Multiple Choice)
4.7/5  (26)
(26)
The Bohr theory of the hydrogen atom predicts the energy difference (in J) between the n = 3 and the n = 5 state to be
(Multiple Choice)
5.0/5  (27)
(27)
The energy of an electron in the hydrogen atom is determined by
(Multiple Choice)
4.9/5  (38)
(38)
a. Use Bohr's equation to calculate how much energy (in J) is needed to promote an electron from the H-atom ground state to the n = 4 level.
b. If a photon provides the energy in (a), what is its wavelength in nm?
(Essay)
4.9/5  (38)
(38)
Who was the first scientist to propose that an object could emit only certain amounts of energy?
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following sets of quantum numbers can correctly represent a 3p orbital?
(Multiple Choice)
4.9/5  (30)
(30)
Who proposed the principle which states that one cannot simultaneously know the exact position and velocity of a particle?
(Multiple Choice)
4.8/5  (35)
(35)
Use the Rydberg equation to calculate the frequency of a photon absorbed when the hydrogen atom undergoes a transition from n1 = 2 to n2 = 4. (R = 1.096776 × 107 m-1)
(Multiple Choice)
4.9/5  (34)
(34)
In the Bohr model of the hydrogen atom, the electron moves in a circular path which Bohr referred to as an orbital.
(True/False)
4.9/5  (34)
(34)
In the quantum mechanical treatment of the hydrogen atom, which one of the following combinations of quantum numbers is not allowed? 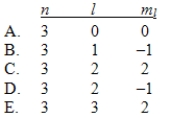
(Short Answer)
4.8/5  (35)
(35)
a. Calculate the momentum of a photon of green light, wavelength 515 nm.
b. If this photon is traveling in a vacuum, what is its "mass"?
(Essay)
5.0/5  (36)
(36)
Platinum, which is widely used as a catalyst, has a work function (the minimum energy needed to eject an electron from the metal surface) of 9.05 × 10-19 J. What is the longest wavelength of light which will cause electrons to be emitted?
(Multiple Choice)
4.8/5  (30)
(30)
A modern compact fluorescent lamp contains 1.4 mg of mercury. If each mercury atom in the lamp were to emit a single photon of wavelength 254 nm, how many joules of energy would be emitted?
(Multiple Choice)
4.8/5  (36)
(36)
Use the Bohr equation to calculate the energy of
a. the largest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
b. the smallest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
(Essay)
4.9/5  (34)
(34)
An electron in the n = 6 level emits a photon with a wavelength of 410.2 nm. To what energy level does the electron move?
(Multiple Choice)
4.7/5  (31)
(31)
Showing 41 - 60 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)