Exam 10: The Shapes of Molecules
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Thionyl chloride is used as an oxidizing and chlorinating agent in organic chemistry. Select the best Lewis structure for SOCl2.
(Multiple Choice)
4.9/5  (34)
(34)
The formal charges on Cl and O in the structure shown for the ClO- ion are, respectively 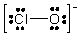
(Multiple Choice)
4.8/5  (49)
(49)
Which one of the following Lewis structures is definitely incorrect? 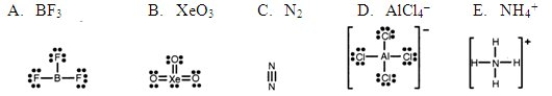
(Multiple Choice)
4.7/5  (36)
(36)
In which one of the following structures does the central atom have a formal charge of +2?
(Multiple Choice)
4.9/5  (37)
(37)
According to VSEPR theory, a molecule with the general formula AX3E2 will have a _____ molecular shape.
(Multiple Choice)
4.8/5  (33)
(33)
According to VSEPR theory, a molecule with the general formula AX2E2 will have a _____ molecular shape.
(Multiple Choice)
4.7/5  (33)
(33)
List the three important ways in which molecules can violate the octet rule, and in each case draw one Lewis structure of your choice as an example.
(Essay)
4.8/5  (29)
(29)
Predict the ideal bond angles around carbon in C2I2 using the molecular shape given by the VSEPR theory.
(Multiple Choice)
4.8/5  (43)
(43)
Use VSEPR theory to decide which one of the following molecules and ions will definitely have at least one 90° bond angle in it. (In each case except water, the central atom is the first one in the formula.)
(Multiple Choice)
4.9/5  (41)
(41)
What is the molecular shape of the thiocyanate anion, SCN-, as predicted by the VSEPR theory? (Carbon is the central atom.)
(Multiple Choice)
4.9/5  (41)
(41)
What is the molecular shape of NOCl as predicted by the VSEPR theory? 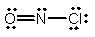
(Multiple Choice)
4.8/5  (38)
(38)
Bond angles of 180° only occur around atoms which display linear molecular geometry.
(True/False)
4.8/5  (31)
(31)
Draw Lewis structures, showing all valence electrons, for:
a. N
b. Br-
c. O2
d. SO42-
(Essay)
4.8/5  (42)
(42)
What is the molecular shape of BeH2 as predicted by the VSEPR theory?
(Multiple Choice)
4.8/5  (38)
(38)
In which one of the following is the best Lewis structure a resonance structure? (central atoms are bold)
(Multiple Choice)
4.8/5  (38)
(38)
In which one of the following species is the central atom (the first atom in the formula) an exception to the octet rule?
(Multiple Choice)
4.8/5  (38)
(38)
In order for a non-cyclic triatomic molecule to be bent, VSEPR theory requires that there must be two lone pairs on the central atom.
(True/False)
4.8/5  (37)
(37)
According to VSEPR theory, a molecule with the general formula AX5 will have a ______ molecular shape.
(Multiple Choice)
4.8/5  (39)
(39)
In formaldehyde, CH2O, both the formal charge and the oxidation number of carbon are zero.
(True/False)
4.9/5  (41)
(41)
Showing 41 - 60 of 109
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)