Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Which of the following properties measures the energy needed to increase the surface area of a liquid?
(Multiple Choice)
4.9/5  (35)
(35)
How do the electrical properties of semiconductors differ from those of metals?
(Essay)
4.9/5  (39)
(39)
Polonium crystallizes in the simple cubic lattice. What is the coordination number for Po?
(Multiple Choice)
4.8/5  (39)
(39)
Select the pair of substances in which the one with the lower vapor pressure at a given temperature is listed first.
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following liquid substances would you expect to have the lowest surface tension?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following atoms should have the greatest polarizability?
(Multiple Choice)
4.9/5  (40)
(40)
All gases can be liquefied at room temperature simply by increasing the pressure on the gas.
(True/False)
4.9/5  (37)
(37)
A temperature increase causes __________________ in the conductivity of a semiconductor.
(Multiple Choice)
4.8/5  (29)
(29)
Octane has a vapor pressure of 40. torr at 45.1°C and 400. torr at 104.0°C. What is its heat of vaporization?
(Multiple Choice)
4.7/5  (35)
(35)
Only molecules which do not have dipole moments can experience dispersion forces.
(True/False)
4.9/5  (36)
(36)
Draw a fully labeled phase diagram (P versus T) of a substance whose solid phase can melt due to applied pressure (i.e., solid is less dense than liquid). Clearly label the triple point and the critical temperature on your diagram.
(Essay)
4.9/5  (39)
(39)
Consider the following phase diagram and identify the process occurring as one goes from point C to point D. 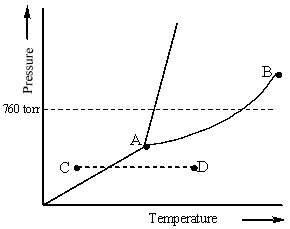
(Multiple Choice)
4.8/5  (31)
(31)
Iron has a body-centered cubic unit cell, and a density of 7.87 g/cm3. Calculate the edge length of the unit cell, in pm. (The atomic mass of iron is 55.85 amu. Also, 1 amu = 1.661 × 10-24 g.)
(Short Answer)
4.8/5  (34)
(34)
Liquid ammonia boils at -33.4°C and has a heat of vaporization of 23.5 kJ/mol. Calculate its vapor pressure at -50.0°C.
(Short Answer)
4.9/5  (33)
(33)
The surface tension of water is lowered when a detergent is present in solution.
(True/False)
4.8/5  (40)
(40)
The energy gap between the conduction band and the valence band is large for
(Multiple Choice)
4.9/5  (32)
(32)
The density of solid sodium chloride, NaCl, is 2.17 g/cm3. Use your knowledge of the sodium chloride lattice to calculate the spacing between Na+ and Cl- nearest neighbors, in cm.
(Atomic masses (amu) are: Na, 22.99; Cl, 35.45. Also, 1 amu = 1.661 × 10-24 g)
(Short Answer)
4.8/5  (43)
(43)
Which of the following terms refers to the resistance of a liquid to flow?
(Multiple Choice)
4.9/5  (36)
(36)
The maximum number of phases of a single substance which can coexist in equilibrium is two.
(True/False)
4.8/5  (43)
(43)
Lead crystallizes in the face-centered cubic lattice. What is the coordination number for Pb?
(Multiple Choice)
4.9/5  (44)
(44)
Showing 21 - 40 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)