Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
The strongest intermolecular interactions between ethyl alcohol (CH3CH2OH) molecules arise from
(Multiple Choice)
4.8/5  (40)
(40)
Iron crystallizes in the body-centered cubic lattice. What is the coordination number for Fe?
(Multiple Choice)
4.8/5  (37)
(37)
Hexagonal close packing of identical atoms occurs when close-packed layers are stacked in an abcabc.... arrangement.
(True/False)
4.8/5  (34)
(34)
Chlorine trifluoride is used in processing nuclear reactor fuel. It has a vapor pressure of 29.1 torr at -47.0°C and its heat of vaporization is 30.61 kJ/mol. At what temperature would its vapor pressure be 107.7 torr?
(Short Answer)
4.8/5  (38)
(38)
Liquid sodium can be used as a heat transfer fluid. Its vapor pressure is 40.0 torr at 633°C and 400.0 torr at 823°C. Calculate its heat of vaporization.
(Multiple Choice)
4.9/5  (52)
(52)
Which of the following pairs of molecules can form hydrogen bonds between them?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following factors contributes to a low viscosity for a liquid?
(Multiple Choice)
4.9/5  (45)
(45)
If the solid form of a pure substance is denser than its liquid form, an increase in pressure will cause the melting point to decrease.
(True/False)
4.8/5  (38)
(38)
The strongest intermolecular interactions between hydrogen sulfide (H2S) molecules arise from
(Multiple Choice)
4.7/5  (44)
(44)
a. Name the two unit cells which occur in close packing of identical atoms.
b. Briefly explain how the two types of close-packed lattices of identical atoms differ, in terms of atomic arrangements.
(Essay)
4.9/5  (39)
(39)
A metal such with a face-centered cubic lattice will have ________________ atom(s) per unit cell.
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following should have the highest boiling point?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following statements about unit cells and packing in solids is incorrect?
(Multiple Choice)
5.0/5  (39)
(39)
In an ionic solid MX consisting of the monatomic ions, M+ and X-, the coordination number of M+ is _______________.
(Multiple Choice)
4.9/5  (42)
(42)
Assuming that atoms are spherical, calculate the fraction of space which is occupied by atoms (i.e., the packing efficiency) in a metal with a simple cubic unit cell.
(Short Answer)
4.9/5  (35)
(35)
A certain solid metallic element has a density 7.87 g/cm3 and a molar mass of 55.85 g/mol. It crystallizes with a cubic unit cell, with an edge length of 286.7 pm. Calculate the number of atoms per unit cell.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following pairs is arranged with the particle of higher polarizability listed first?
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the following quantities is generally not obtainable from a single heating or cooling curve of a substance, measured at atmospheric pressure?
(Multiple Choice)
4.8/5  (38)
(38)
Consider the phase diagram shown below. 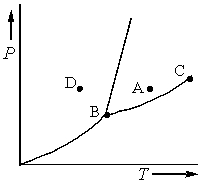 a. What phase(s) is/are present at point A?
b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.
a. What phase(s) is/are present at point A?
b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.
(Essay)
4.7/5  (46)
(46)
Some of the information obtained from the heating or cooling curve of a substance can also be found on a phase diagram of that substance.
(True/False)
4.8/5  (33)
(33)
Showing 41 - 60 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)